OCR A 2.2.1 Electron Structure
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
atomic orbitals
-a region around the nucleus that can hold up to 2 electrons
-with oppo spin
shells
-energy levels
-energy increases as shell number increases
principal quantum number
-(n)
-shell/energy level number
how many subshells in electron shell
4
electron subshells
-s; 1 orbital, holds 2 e (1s)
-p; 3 orbitals, holds 3 × 2 = 6 e (2s, 2p)
-d; 5 orbitals, holds 5 × 2 = 10 e (3s, 3p, 2d)
-f; 7 orbitals, holds 7 × 2 = 14 e
relationship between shell number and nucleus
higher the shell number, further away it is from nucleus and has higher energy
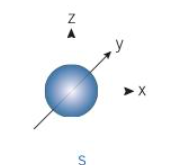
s orbital shape
-1 orbital
-e cloud within shape of sphere
-e move anywhere within sphere
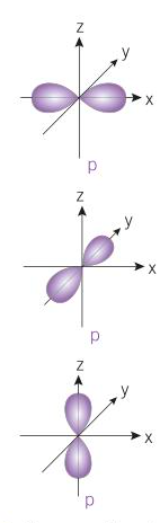
p orbital shape
-3 orbitals
-electron cloud within shape of dumbbell
-3 separate dumbbells at right angles to each other
-e move anywhere within shape
spin pairing
-when 2 e occupy 1 orbital, they spin in oppo direction
-as e are -ve they repel
-oppo spin counteracts repulsion
orbital filling rules
-fill in order of increasing energy; 1s2 2s2 2p6 3s2 3p6 4s2 (4s fills before 3d)
-orbital w/ same energy singly filled, then dbly; prevents repulsion between paired e
electron config of ions
-when filled 4s energy level is higher than 3d
-4s empties before 3d