Enzymes
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
What are enzymes? What are their functions in eye?
Proteins that optimize the rates of biochemical reactions in cellular tissues.
They are involved in visual transduction, generation of IOP, maintenance of clear cornea, destruction of bacteria in tear film, development of lens fibers, and several other functions in the eye.
What is the influence of an enzyme on activation energy and rate of reaction?
Enzymes increase the rate of a reaction. They act by lowering the activation energy to
convert a reactant into a product.
What is the principal enzyme driving the reaction? How does substrate and product concentration influence the activity of enzyme?
for enzyme catalyzed reactions, a large amount of substrate (A) will drive the reaction to form product (C) and vice versa because these reactions are usually reversible.
How does the concentration of enzyme change with time in an enzymatic reaction?
enzyme becomes saturated very early in time with substrate due to the formation of the enzyme-substrate complex [ES].
This complex remains at a fairly steady level. The enzyme is always returned to its original form at the end of each reaction.
Therefore, the concentration of free enzyme might change initially but the total enzyme concentration (free and bound) would remain relatively constant over the course of the reaction, assuming no enzyme degradation or synthesis.
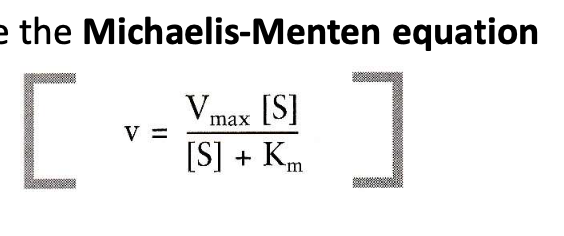
What is Michaelis Menten equation? Identify the terms and its application
maximum velocity (Vmax) and dissociation constant (Km) of an enzyme.
Its application includes measuring the rate properties of an enzyme (how fast they catalyze reactions) and comparing the relative affinity (stickiness) of different substrates for the same or different enzymes.
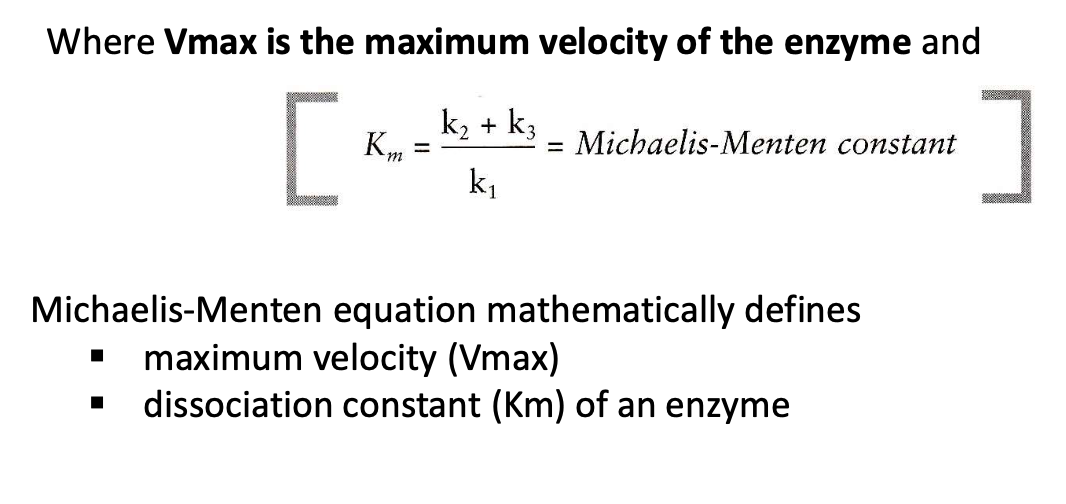
What is the significance of Michaelis Menten constant? How is it related to substrate concentration?
a lower Km indicates a greater affinity of the substrate and enzyme, provided that k3 is small enough.
Km is = to substrate concentration when velocity is 1/2 Vmax
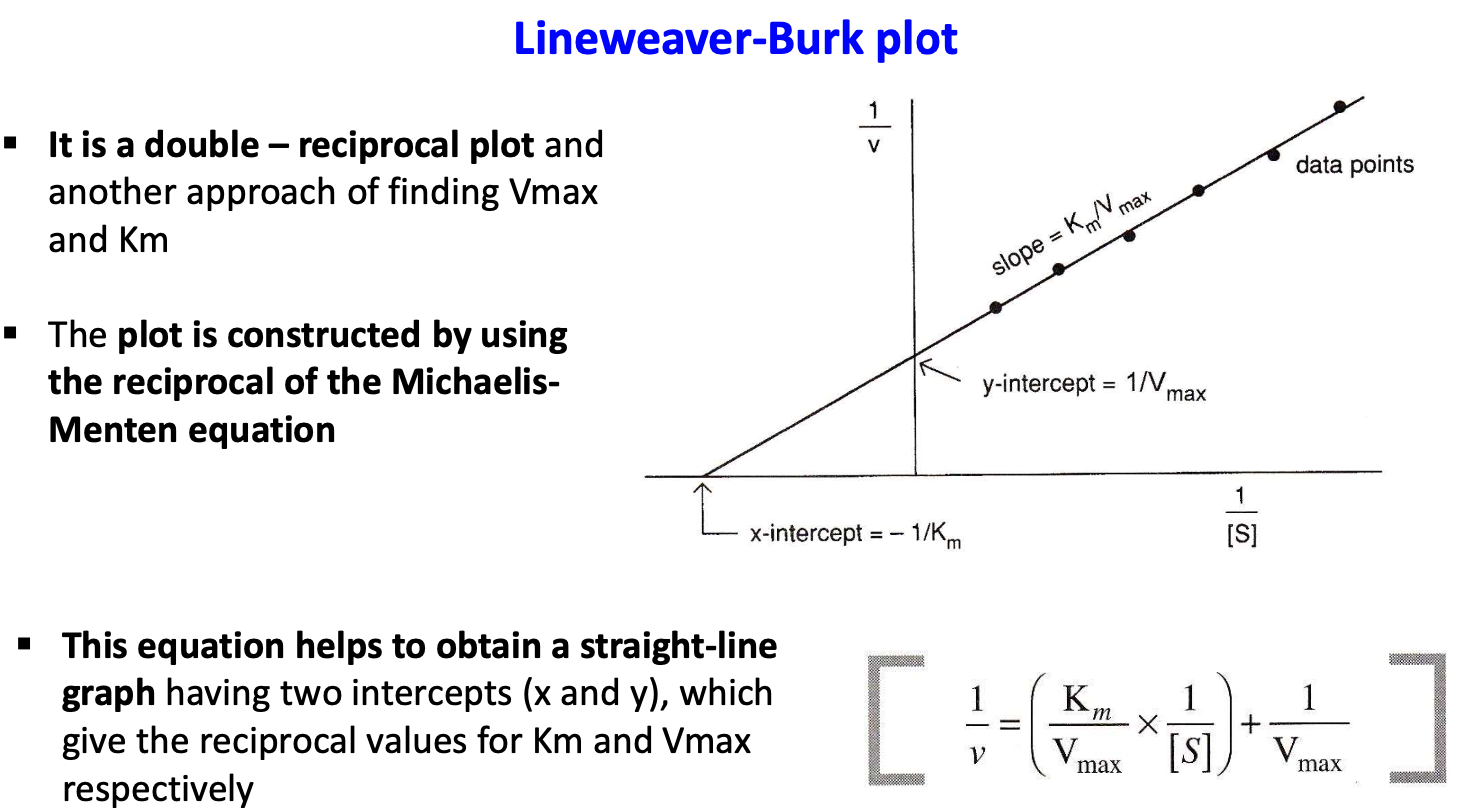
What is Lineweaver Burk plot?
The Lineweaver-Burk plot is a double - reciprocal plot and another approach for finding Vmax and Km
(reciprocal of MM equation)
What are allosteric enzymes? How does the conformation of these enzymes change?
Enzymes whose kinetics is influenced by substrates bound to the enzyme at locations other than the active site.
How does the conformation of allosteric enzymes change?
They change from a T-form (tense) to an R-form (relaxed)
When the number of occupied active sites increase, an overall conformational change in the enzyme occurs, making it more favorable to increased binding of the substrate to remaining active sites.
How does binding of an activator influence the allosteric enzyme?
The binding of an activator to an allosteric enzyme at a site away from the active site can facilitate the change from the T to the R form at lower substrate concentrations. This decreases the apparent Km (Kapparent) and increases the velocity.
What is the limitation of Lineweaver Burk plot?
The Lineweaver-Burk plot becomes non- linear with allosteric enzymes, making it impossible to determine Kapp.
What is the relation of Kapp to reaction velocity?
The relationship between Kapp and reaction velocity in allosteric enzymes is complex and influenced by the binding of activators or inhibitors, making the simple Michaelis-Menten relationship non- applicable.
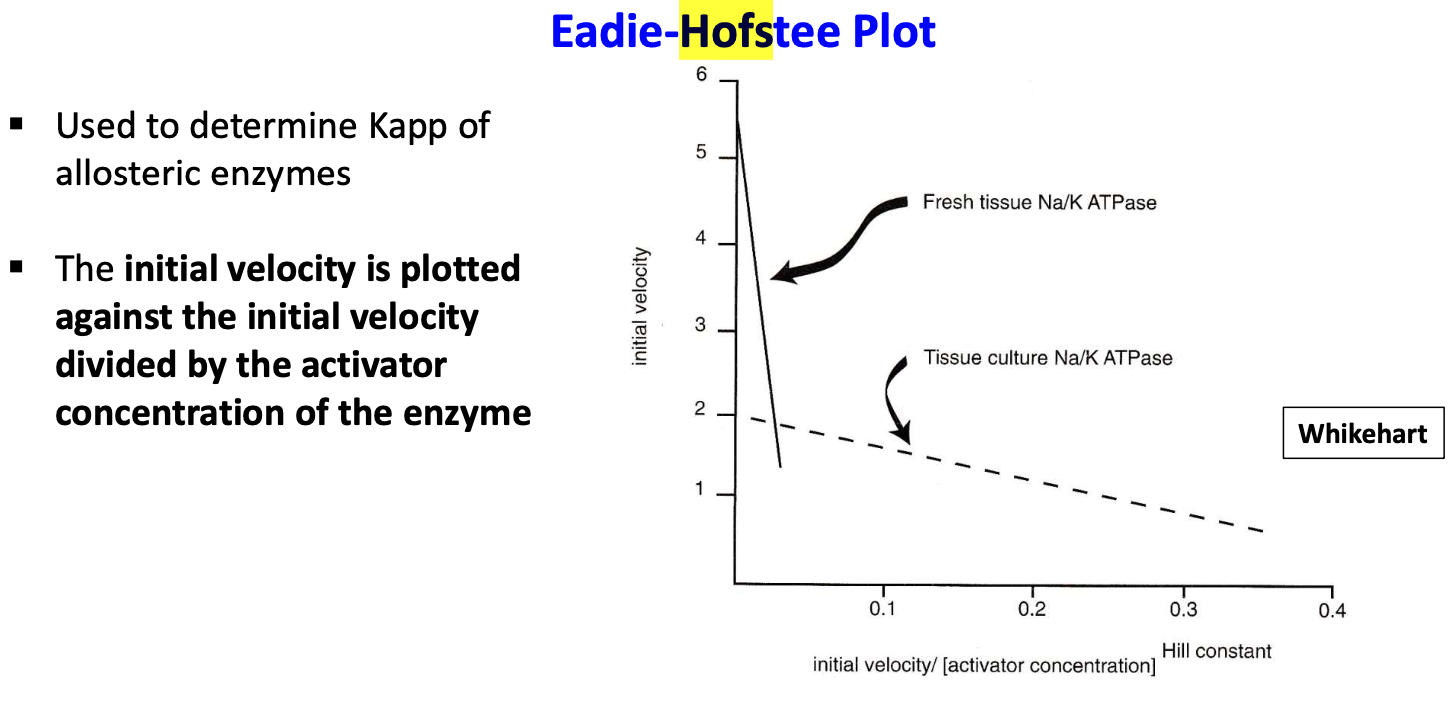
What is a Eadie Hofstee plot? When is it used and what is its application?
used to determine Kapp of allosteric enzymes. involves plotting the initial velocity against the initial velocity divided by the substrate concentration (S) or activator concentration(A)
It can be used to determine sodium activation for Na, K-ATPase
What are enzyme inhibitors and name the three mechanisms by which they inhibit enzyme?
they negatively influence the rates of enzymes.
Michaelis-Menten enzymes are inhibited by one of three mechanisms:
- competetive
- noncompetetive
- uncompetitive
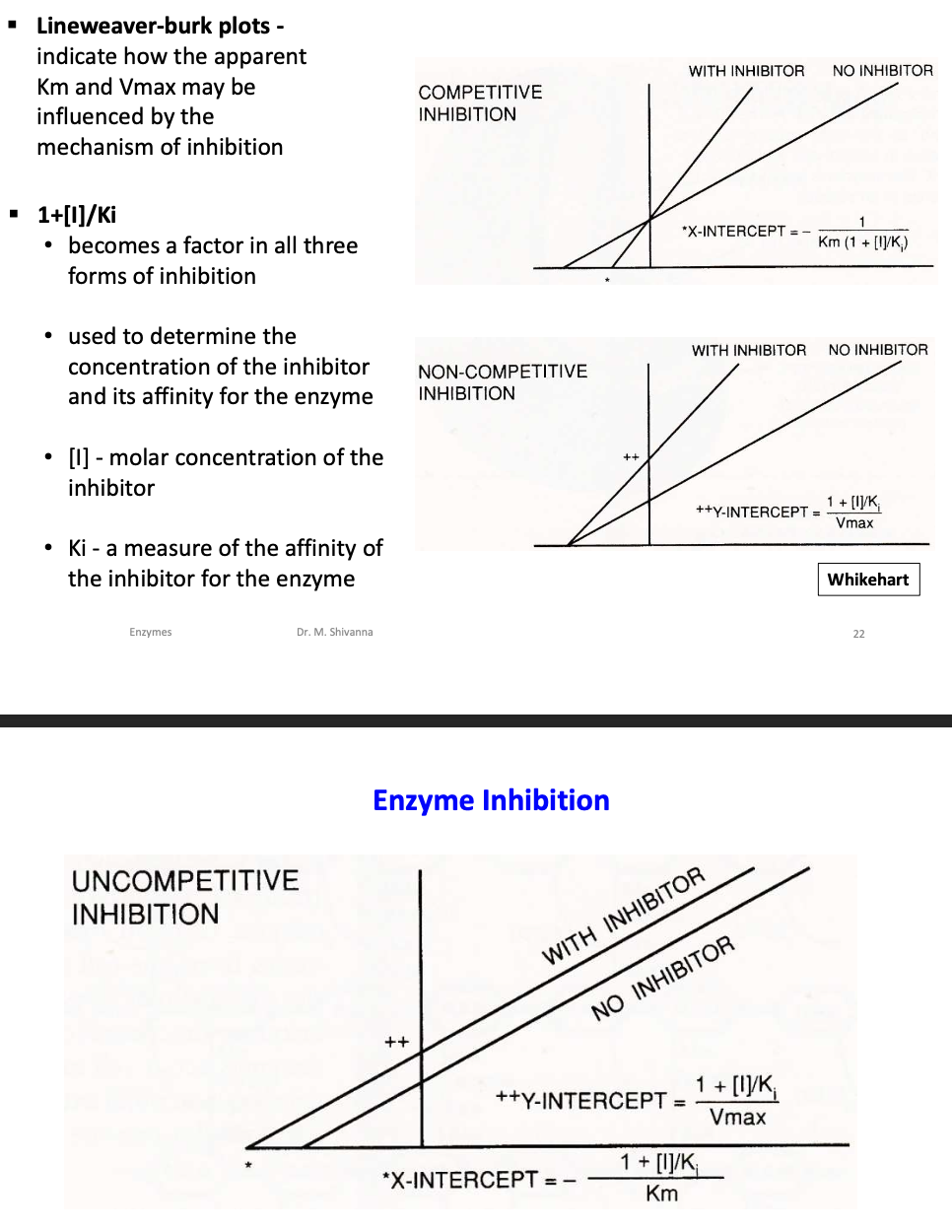
What is the factor that is used to determine concentration of inhibitor and its affinity for the enzyme?
1+[I]/Ki
[I]-molar concentration of the inhibitor
Ki-a measure of the affinity of the inhibitor for the enzyme
What mechanism is involved in inhibition of aldose reductase?
uncompetitive or noncompetitive, depending on the inhibitor used.
What is the influence of an inhibitor on allosteric enzyme?
prevents the change from T to the R form even at high substrate concentrations, decreasing velocity
What is feedback inhibition?
the end product of the series act as an inhibitor for the first reaction in the series
What is lysozyme and where is it found? Give its concentration in tear fluid.
Lysozyme is an enzyme of the pre corneal tear film. It is a globular protein with a molecular with of 14k.
It's concentration is 1.3 mg/ml
What is the influence of lysozyme on peptidoglycan coat of bacteria?
Lysozyme helps destroy gram (+) bacteria that have an outer coat of peptidoglycan.
It hydrolyzes the glycan component of the bacteria by breaking the β1→4 glycosidic bond of the oxygen bridge between the repeating glycan units of N-acetylmuramic acid (NAM) and N-acetylglucosamine (NAG).
The bacteria then bursts open, cannot contain high osmotic pressure with only plasma membrane
Identify the site where lysozyme acts to break the peptidoglycan coat.
Lysozyme acts at its active site, a cleft on the outside or or small cavern on the inside of the structure of protein.
Where does the bacterial coat fit on lysozyme?
A portion of the bacterial peptidoglycan fits in a groove on the outer surface of the enzyme that contains the active site.
What does lysozymal activity indicate? What is the assay used for ?
An important analytical indicator of tear dysfunction.
It assesses the productivity of the lacrimal glands and status of aqueous deficiency.
Where is Na, K-ATPase located? What are its two functions related to the eye?
in the plasma membrane of a variety of cells.
- corneal hydration
- production of aqueous fluid
Identify the structural components of Na, K-ATPase. Give the catalytic reaction catalyzed by the enzyme
4 polypeptide chains, two α and two β chains.
The catalytic reaction is coupled to an ion transport where inorganic phosphate is bound to one of the a-subunits, supply energy to transport 3 Na+ out, 2 P+ in
Where is Na, K-ATPase located in the cornea and ciliary epithelium?
cornea: in the plasma membrane of cells,
ciliary epithelium: in the NPE cells to produce aqueous fluid
What is the consequence of lack of Na, K-ATPase activity on cornea and intraocular pressure?
cornea: Na+ ions and water continuously enter the corneal storm, causing swelling and become opaque.
ciliary epithelium: there would be a reduction in the pumping of Na+ ions, reducing the osmotic flow of water and lowering the IOP
Where does lactate dehydrogenase operate and where is it located?
in the Cytoplasm, at the metabolic junction of aerobic and anaerobic metabolism
What are the substrates for lactate dehydrogenase? Give the reaction for conversion of pyruvate to lactate?
pyruvate and coenzyme NADH.

three kinds of polypeptide subunits identified in lactate dehydrogenase? What type of lactate dehydrogenase is found in retina and cancer cells?
H (heart)
M (muscle)
K (subunit in cancer cells)
K4 or LDHk is in retina and cancer cells
What influences the ability of lactate dehydrogenase towards aerobic or anaerobic pathway?
the kinetic properties of each LDH enzyme
quick energy: anaerobic
fuel efficient: aerobic
How does LDH influence corneal epithelial cells under conditions of low partial pressures of oxygen?
they must shunt their carbohydrate metabolism to lactate production via LDH. this supports the cells at low pressures (15-20 mm Hg)
Different isozymes of LDH in human cornea and their percentages?
H2M2: 25%
HM3: 65%
M4: 10%.
Two isozymes of LDH in human lens?
HM3 and M4 LDH isozymes
influence of LDH on retina?
LDHk opens the pathway to lactate when no further pyruvate can be driven through the aerobic pathway.
Helps in the rapid metabolism of glucose since the energy demands in photoreceptors are high.
Warburg effect
Production of lactate in retina higher than in any other aerobic tissue
Under what disease conditions is aldose reductase activated? What reactions does it catalyze?
cataract formation in patient with diabetes and galactosemia.
It catalyzes the conversion of glucose to sorbitol and galactose to galacitol.
What are polyols? What is their influence on lens fiber cells?
an osmotic imbalance in lens fiber cells which causes them to swell and burst since they can't exit - Sorbitol and Galactitol
When does aldose reductase enzyme gets activated in diabetes?
when blood sugar levels approach 20mM for sustained periods.
What are the different sites on aldose reductase enzyme? What is the nature of inhibitory site on the enzyme?
an inhibitor site on a lipophilic enzyme.
The site associates with a variety of sat soluble substances that act as inhibitors
three inhibitors identified for aldose reductase enzyme? What are their properties?
Quercetin
Sorbinil
Tolrestat
none approved by FDA
Quercetin
too weak inibitor
Sorbinil
high degree is hypersensitive reactions
Tolrestat
recently tested
the mechanism involved in inhibition of aldose reductase enzyme?
either uncompetitive or noncompetitive, depends on the inhibitor used
What are MMPs and what functions do they regulate?
MMPs are protein hydrolyses. They breakdown ECM or noncellular tissue architecture, control growth/development, tissue maintenance, tissue reformation/detioration
the three main classes of MMPs and give their substrates and their roles?
- collagenases: break down collagen
- gelatinases: breakdown type IV and V collagens
- stromolysins: break down non collagenous ECM proteins
How do MMPs induce myopia? What is the influence of MMPs on scleral proteins
controlled gelatinize A (MMP2 plays a role in lengthening the globe when myopia is induced. (in chicks)
Occurs as a result of partial digestion of scleral proteins, and new proteins formed establish new scleral length.
How does MMP2 and TIMP2 mRNA levels vary during myopia and after recovery?
During induced myopia: mRNA used to synthesize gelatinase A increased by 125%
mRNA used to synthesize TIMP-2 increased by 75%.
After deprivation for 24hrs, mRNA: 50% TIMP-2: 12%
What are the two types of MMP inhibitors used to limit corneal ulceration?
TIMP: naturally secreted polypeptide with MMPs
B mercaptomethyl-Leu-Phe-Ala (SIMP, synthetic inhibitor of metalloproteinase)
What is the effect on corneal ulceration in the absence of inhibitor?
tissues not exposed to SIMP achieved clinical score of 3 or worse after four days, indication ulceration.