Microscopy, Spectroscopy, & Spectrometry
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
An optical instrument that uses a lens or a combination of lenses to magnify and resolve the fine details of an object.
Microscope
A two monocular compound microscope to present a 3D image to the viewer, who looks through both eyepiece lenses. Particularly useful for evidence that does not require a high magnification (10x-125x).
Stereomicroscope
An optical microscope that uses two or more lenses to achieve higher magnification, typically providing a magnification range of 40x to 1000x.
Compound microscope
What two systems is the compound microscope composed of?
Mechanical and optical system
A type of microscope that allows for the simultaneous viewing of two specimens side by side, enabling comparisons between them. It is often used in forensic analysis to compare samples like hair or fibers.
Comparison microscope
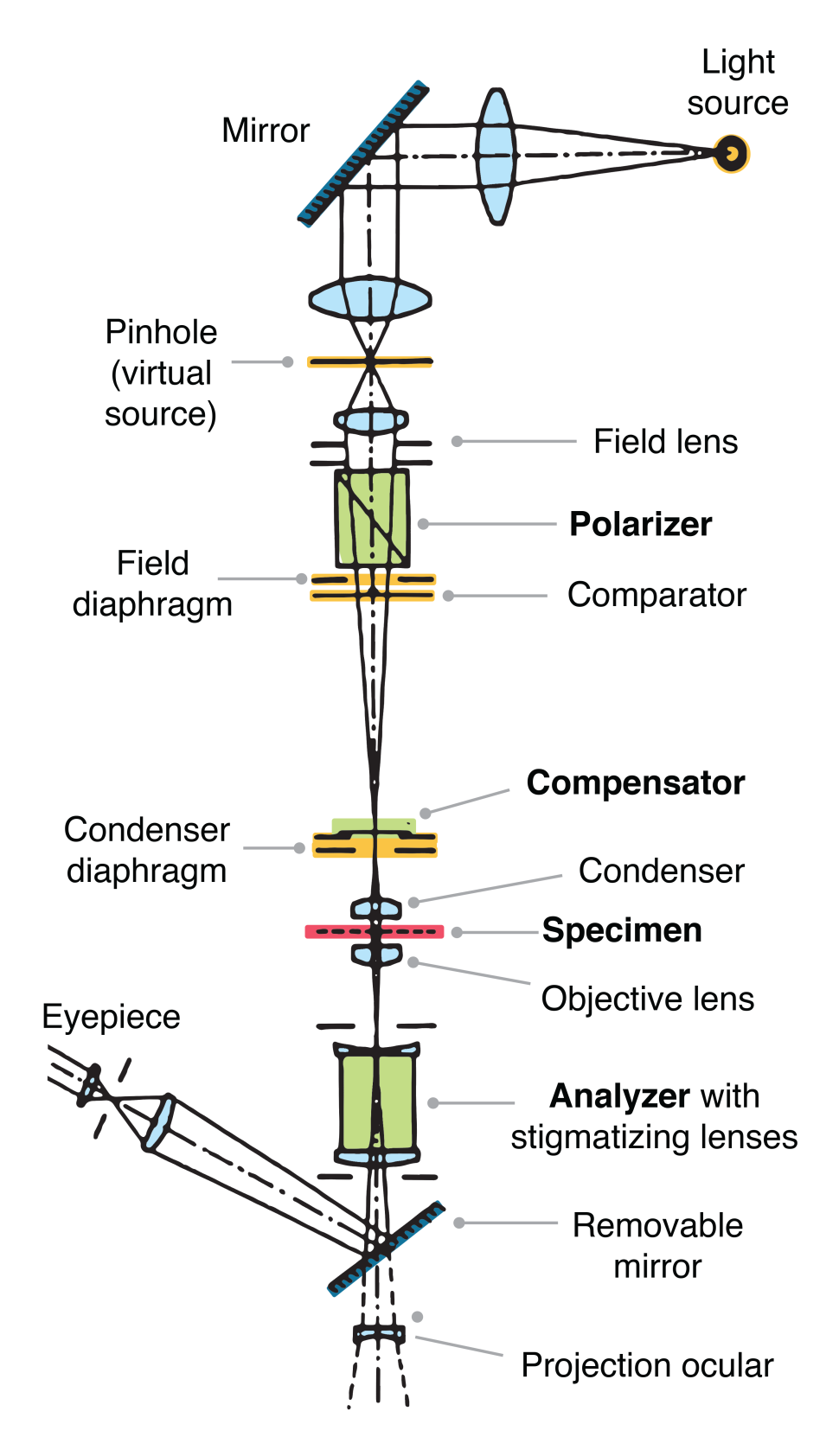
A technique examining the interaction of plane-polarized light with matter. Can be used to study birefringent materials. Light waves normally vibrate perpendicular to their travel path.
Polarized microscopy
Bombards a specimen with a beam of electrons instead of light to produce a highly magnified image from 100x to 100,0000x, providing detailed information about the surface morphology and composition of the sample.
Scanning Electron Microscope (SEM)
The study of the absorption and emission of light and other radiation by matter. Involves splitting of light (electromagnetic radiation) into wavelengths.
Spectroscopy
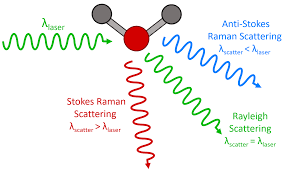
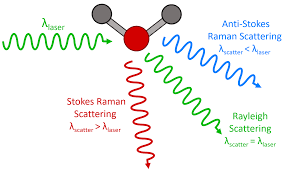
A technique that utilizes a tiny portion of light scattered by the sample, giving it a different frequency. This moves the photon to an exciting-rotationa-vibrational-electronic state where photons are emitted from sample. It provides information on molecular vibrations and can be used for chemical composition analysis (a ____ spectrum).
Raman spectroscopy
A technique that measures how much infrared light is absorbed by a sample at different wavelengths, providing insights into molecular structure and composition, a “chemical fingerprint”.
FTIR Spectroscopy
The measurement of the interaction between light and matter and the reactions and measurements of radiation intensity and wavelength.
Spectrometry
Separates the different components of a mixture.
Gas Chromatography
Further separates the components based on their mass-to-charge ratio.
Mass Spectrometry
What are these?…
1. Place a piece of activates charcoal (c-strip) into the sample overnight
2. Heat sample overnight
3. Volatile compounds from the sample enter the headspace of the container and stick to the c-strip
4. Remove c-strip and elute with a solvent
Steps for fire debris sample preparation
What are the two columns for gas chromatography?
Packed and capillary
These columns are made out of glass, making them more narrow than packed columns. They allow for better separation and increased sensitivity in gas chromatography.
Capillary
What does gas chromatography produce?
Chromatogram
??? = ( substance area / total area ) x 100
Percent composition
What does Mass Spectrometry produce?
Mass spectrum
What does it mean if something is bombarded with ions?
Positively charged