Chemistry Finals Review 2024 (Atomic Structure & Electron Configurations)
1/13
Earn XP
Description and Tags
Mr. Janick 1st hr
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Protons
Found in Nucleus, 1+ charge per proton, 1 AMU atomic mass unit. Number of protons = the atomic number
Neutrons
Found in Nucleus, 0 charge, 1 AMU (atomic mass unit)
The mass of an atom (mass number [?+?])
Protons + Neutrons
Electrons
Found in Electron Cloud (Orbit, Shell), 1- charge per electron, 0 AMU atomic mass unit. Number of electrons = the number of protons in a neutral atom. Electrons determine the chemical bonding between atoms
Nucleus
Center of the atom and has the protons and neutrons
Atomic Number
Number of protons
Ions
Gained or lost electrons
Cation
Lose electrons. Has a positive charge
Anion
Gains electrons. Has negative charge
Isotope
Atoms of the same element (type) that has different number of neutrons (Changes the Mass)
Calculate protons, neutrons and mass (Example problems)
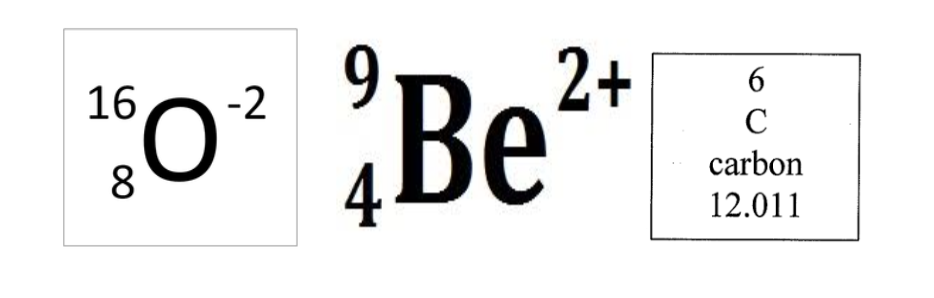
Bohr Energy Levels (#)
7
How many Electrons per energy level?
2, 8, 18, 32, (32, 32, 32)
S, P, D, F electrons
S = 2
P = 6
D = 10
F = 14