IUPAC Nomenclature of Alkanes - Organic Chemistry
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
1 Carbon
meth
2 Carbons
eth
3 Carbons
prop
4 Carbons
but
5 Carbons
pent
6 Carbons
hex
7 Carbons
hept
8 Carbons
oct
9 Carbons
non
10 Carbons
dec
2 Substituent Groups
di-
3 Substituent Groups
tri-
4 Substituent Groups
tetra-
5 Substituent Groups
penta-
6 Substituent Groups
hexa-
7 Substituent Groups
hepta-
8 Substituent Groups
octa-
Common Alkane Groups
Common Formula: CnH2n+2
1) Methane: CH4
2) Ethane: C2H6
3) Propane: C3H8
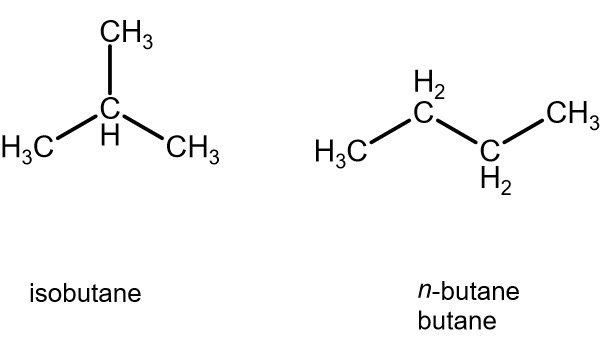
4) Butane: C4H10
5) Isobutane: C4H10 (branched)
Alkyl Group
Formed by the removal of H from alkane chain
Naming: replace -ane with -yl
Prefix -iso for constitutional isomers that are branched
Carbon Structures [4]
Primary: most reactive
Quaternary: least reactive
1) Primary: Carbon attached to 1 other carbon
2) Secondary: Carbon attached to 2 other carbons
3) Tertiary: Carbon attached to 3 other carbons
4) Quaternary: Carbon attached to 4 other carbons