Exam 4 Review
1/357
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
358 Terms
What is the definition of epilepsy? At least . involuntary & unprovoked seizures occurring more than 24 hours apart.
2
What are the most common causes of epilepsy in the US?
Neurocysticercosis & HIV
Genetic mutations & developmental defects
Hyperkalemia & hypercalcemia
Head trauma & stroke
4
Which of the following are seizure triggers? Select all that apply.
Photostimulation
Diabetes
Sleep deprivation
Hyperventilation
1,3,4
Please identify at least 3 drugs that may lower the seizure threshold.
Bupropion, tramadol, TCAs, clozapine, lithium, cefepime, carbapenem, fluoroquinolones, recreational drugs
Which of the following characteristics apply to a focal seizure? Select all that apply.
The symptoms are only motor (ex. muscle contraction)
Starts in both hemispheres of the brain
Usually unilateral movements
Movements are often symmetric
The patient may or may not remain conscious.
3,5
Which of the following lab values may be transiently elevated immediately after a seizure?
Ammonia
Lactate dehydrogenase
Prolactin
Troponin
3
What is the definition of status epilepticus? Continuous seizure activity for at least . minutes.
5
A patient in the ED (70 kg) has status epilepticus and the resident physician asks what to give. What is the first line recommendation & dose?
Lorazepam 0.1 mg/kg
Which of the following statements is true regarding lorazepam? Select all that apply.
The brand name is Valium.
It is metabolized to an active metabolite.
The IV formulation contains propylene glycol.
It has delayed uptake into the brain due to low lipophilicity
3,4
According to the 2018 AAN/AES guidelines, which of the following is NOT a first line agent for adults with new-onset focal epilepsy or unclassified generalized tonic-clonic seizures?
Lamotrigine
Levetiracetam
Vigabatrin
Zonisamide
3
According to the 2018 AAN/AES guidelines, which of the following are preferred first line agents in a geriatric patient with new-onset focal epilepsy? Select all that apply.
Ethosuximide
Lamotrigine
Valproic acid
Gabapentin
2,4
According to the 2018 AAN/AES guidelines, what is the drug of choice of absence seizures for a young, female patient?
Ethosuxamide
Which of the following AED is correctly matched with its black box warning?
Perampanel - rash (SJS/TEN/DRESS)
Lamotrigine -severe psychiatric and behavioral reactions
Phenytoin - cardiovascular effects with IV infusion
Lacosamide - aplastic anemia & agranulocytosis
3
Which of the following AED is correctly matched with its brand name?
Phenytoin - Keppra
Lacosamide - Vimpat
Carbamazepine - Fycompa
Ethosuximide - Lamictal
2
Which of the following is correct regarding levetiracetam?
It is a strong CYP450 inducer.
It is completely renally cleared.
It is useful in bipolar disorder.
It must be taken every 6 hours.
2
Which AED should not be used in a patient with a sulfa allergy?
Zonisamide
Which of the following is not eliminated renally?
Levetiracetam
Gabapentin
Lorazepam
Topiramate
3
Which of the following may be useful in bipolar disorder (is not associated with changes in behavior as a side effect)?
Lamotrigine
Levetiracetam
Gabapentin
Ethosuximide
Pregabalin
1
What are the three black box warnings for valproic acid?
Increased risk of hepatotoxicity, increased risk of pancreatitis, risk of teratogencity
In what patient population would you be hesitant to recommend lacosamide?
Acute psychosis
Pre-existing cardiac conduction problems
Chronic kidney disease
Pre-existing affective disorders
2
Which of the following statements are true regarding topiramate? Select all that apply.
It is completely metabolized by the liver.
It may also be used for migraine and weight loss.
A common side effect is difficulty concentrating.
It does not require renal dose adjustment.
2,3
In which of the following patient populations is it recommended to screen for the HLA-B*1502 allele prior to starting carbamazepine?
Asian ancestry
African americans
Caucasians
Latino americans
1
Which of the following is not an ADE of phenytoin?
Alopecia
Gingival hypertrophy
Purple glove
Nystagmus
1
Which AED is being used off-label to treat alcohol withdrawal?
Phenytoin
Phenobarbital
Carbamazepine
Topiramate
2
Which AED is a CYP450 inhibitor?
Carbamazepine
Phenytoin
Phenobarbital
Valproic acid
4
Which of the following AEDs require serum drug monitoring?
Levetiracetam
Valproic acid
Zonisamide
Gabapentin
2
When should a patient with well-controlled seizures be seen for follow-up?
Every month
Every year
Every 3 months
Every 2 weeks
2
Please complete this sentence: A patient’s AED may be discontinued once they are seizure-free for . years.
2
Which of the following are receptors for pain?
a) Krause bulbs
b) Meissner’s corpuscles
c) Free nerve endings
d) Ruffini endings
c
[Free nerve endings are the receptors for pain, heat and cold. Krause bulbs and Meissners corpuscles play an important role in mediating touch; Ruffini endings play an important role in mediating sensory inputs from ‘pressure’ sensations; Transduction of signals from these receptors to nerves occurs via activation of GPCRs (G-protein coupled receptors) and specific ion channels such as TRPV1 i.e. transient receptor potential vanilloid 1]
What fibers are responsible for conducting pain?
a) Aß
b) Aδ
c) Aα
d) Aθ
b
[Remember ‘Aδ’ fibres are relatively rapid transmitting (myelinated) fibers (~0.5 seconds). They mediate sharp, well localized pain and produce rapid spinal reflex to withdrawal from stimulus; In contrast, ‘C’ fibers consist of slow transmitting (unmyelinated) fibers (~several seconds). They mediate more prolonged, burning pain.]
Which site in the spinal cord is the primary site of integration of peripheral pain fibers with the ascending pain pathway?
a) Rostral ventral medulla
b) Periaqueductal grey matter
c) Autonomic ganglia
d) Dorsal horn
d
[Dorsal horn of the spinal cord is part of both the ascending and descending pain pathway. It is located in the spinal cord; Rostral ventral medulla, and periaqueductal grey matter play a role in the pain pathway but are not part of the spinal cord; Autonomic ganglia are not located in the spinal cord and do not play a role in the transmission of pain]
All of the following brain regions in the brain play a role in mediating pain and are part of the ascending pain pathway (SELECT ALL THAT APPLY)
a) Amygdala
b) Thalamus
c) Periaqueductal grey matter
d) Somatosensory cortex
a, b, d
[Free nerve endings, dorsal horn of the spinal cord , thalamus, somatosensory cortex, prefrontal cortex, anterior cingulate cortex, insular cortex and amygdala are all part of the ascending pathway of the brain; In contrast, periaqueductal grey matter is part of the descending pain pathway]
Which of the following brain regions is part of the descending pain pathway?
a) Hypothalamus
b) Thalamus
c) Periaqueductal grey matter
d) Cerebellum
c
[Periaqueductal grey matter is the only brain region which is part of the descending pain pathway; thalamus plays a role in the ascending pain pathway; cerebellum plays a role in maintaining balance and gait, eye movements, and movements in space and time; hypothalamus plays a role in temperature regulation, regulation of water intake and appetite, regulation of pituitary, and regulation of circadian rhythm.]
Which of the following statements is true regarding the descending pain pathway?
a) It increases cardiovascular response to pain
b) It modulates the experience of pain
c) It originates in the dorsal horn of the spinal cord
d) It terminates in the periaqueductal grey matter
b
[Descending pain pathway play a role in modulating the experience of pain; it originates in the cortex; Emotional state can significantly influence experience of pain via the descending pathway; Norepinephrine and serotonin play an important role in the descending pain pathway]
The amygdala plays a role in emotional processing of pain
a) True
b) False
a
[The amygdala and prefrontal cortex play a role in emotional processing of pain; the thalamus plays a role in filtering sensory input, while the somatosensory cortex plays a role in determining intensity and localization of pain]
Which of the following are non-pharmacological approaches to treat pain? (SELECT ALL THAT APPLY)
a) Transcranial magnetic stimulation
b) Transcranial direct stimulation
c) Taser therapy
d) Cognitive behavioral therapy
a, b, d
[Transcranial magnetic stimulation, transcranial direct stimulation and cognitive behavioral therapy are some of the non-pharmacological approaches used to treat pain; Tasers are used by police to incapacitate suspects]
Which of the following are endogenous opioid peptides? (SELECT ALL THAT APPLY)
a) Endorphin
b) Enkephalin
c) Dynorphin
d) Substance P
a, b, c
[Endorphin, enkephalin, dynorphin are endogenous opioid; Substance P is not an endogenous opioid]
Dynorphin preferentially binds to which of the endogenous opioid receptors?
a) µ
b) κ
c) δ
d) α
b
[Dynorphin binds to kappa opioid receptors; endorphins preferentially bind to mu opioid receptors; enkephalins preferentially bind to delta opioid receptors; alpha receptors bind to epinephrine and norepinephrine]
What is the effect of endogenous opioid receptor activation on potassium ions?
a) They move into the cell
b) They move out of the cell
c) No movement
b
[Movement of potassium ions out of the cell causes hyperpolarization and decreases the cells neuronal potential resulting in decreased firing; Activation of mu opioid receptors has the following effects: Inhibits adenylate cyclase (cAMP), block entry of Ca2+ via voltage-gated calcium channels, and promotes exit of potassium ions from the cells; together all of these result in inhibition of the neurons on which these receptors are located]
What is the effect of endogenous opioid receptor activation on neurotransmitter release?
a) Neurotransmitter release is decreased
b) Neurotransmitter release is increased
c) No change in neurotransmitter release
a
[Activation of endogenous opioid receptors results in decreased neurotransmitter release]
Opioid receptors located on which of the following pathways are responsible for addiction to opioids?
a) Tuberoinfundibular
b) Mesocorticolimbic
c) Nigrostriatal
d) Ascending
b
[The mesolimbic or mesocorticolimbic pathway is also known as the reward pathway; Mu opioid receptors located on the mesocorticolimbic pathway re responsible for addiction to opioids; Opioids indirectly increase activity of dopamine neurons which form this pathway by inhibiting GABA interneurons which normally have an inhibitory effect on the mesolimbic dopaminergic neurons; Mu opioid receptors located on the ascending pathway are responsible for modulation of pain; Mu opioid receptors do not have clinical effects on the Tuberoinfundibular (plays a role in regulating prolactin) and Nigrostriatal dopamine tracts (plays a role in regulating voluntary movement)]
Stimulation of mu opioid receptors in which of the following is responsible for vomiting associated with opioid painkillers
a) Respiratory tract
b) Gut
c) Chemoreceptor trigger zone (CTZ)
d) Vascular
c
[Remember CTZ is the main vomiting center and thus stimulation of opioid receptor sin the center results in inducing vomiting; Stimulation of opioid receptors in the respiratory tract help in suppression of cough; Stimulation of opioid receptors in the gut result sin constipation; stimulation of mu opioid receptors in the vasculature results in vasodilation; stimulation of opioid receptors in the heart and blood vessels helps decrease the preload and thus is used after a myocardial infarction (heart attack); stimulation of opioid receptors located on mast cells results in release of histamine which results in itching associated with mu opioid medications like morphine, oxycodone]
A mixed agonist-antagonist is a compound that
a) Blocks some or all of the opioid receptor subtypes
b) Blocks some opioid receptors and activates other type of opioid receptors
c) Activates an opioid receptor to effect a submaximal response
d) Activates some or all opioid receptor subtypes and does not block any opioid receptor
b
[A mixed agonist-antagonist is a compound that blocks some opioid receptors and activates other type of opioid receptors (e.g. Pentazocine); An antagonist blocks some or all of the opioid receptor subtypes (e.g. naloxone); Partial agonist activates an opioid receptor to effect a submaximal response (e.g. buprenorphine); An agonist activates some or all opioid receptor subtypes and does not block any opioid receptor (e.g. morphine, methadone).
Which of the following is a strong/potent opioid receptor agonist?
a) Tramadol
b) Fentanyl
c) Codeine
d) Buprenorphine
b
[you should be able to identify potent opioid agonists like morphine, heroin, fentanyl as they can cause acute opioid intoxication and death; Tramadol is a an example of a weak agonist; Codeine is an example of moderate agonist; Buprenorphine is an example of partial agonist].
Which of the following are effects seen with pain killers that activate the mu opioid receptors? (SELECT ALL THAT APPLY)
a) Analgesia
b) Sedation
c) Pupillary constriction
d) Diarrhea
a, b, c
[Pain killers that activate mu opioid receptors produce analgesia, sedation, and pupillary constriction; Pupillary constriction is in fact one of the sign and symptoms of opioid overdose; Diarrhea is a sign of opioid withdrawal; Opioids are used for the treatment of traveller’s diarrhea; Remember mu opioid agonist like morphine, oxycodone cause constipation; constipation is major problem in cancer patient being treated with potent opioid agonist to treat cancer-associated pain]
Respiratory suppression seen with mu opioid agonist overdose is due to stimulation of mu opioid receptors located in the
a) Respiratory tract
b) Medulla
c) Chemoreceptor trigger zone
d) Vascular
b
[Remember the respiratory center is located in the medulla (lectures in the first week); stimulation of mu opioid receptors in the medullar causes inhibition of respiratory center; inhibition of the respiratory center is the principal cause of death in case of heroin or fentanyl or morphine overdose; Stimulation of mu opioid receptors in the respiratory tract helps to suppress cough; stimulation of mu opioid receptors in the chemoreceptor trigger zone produces vomiting and nausea; finally, stimulation of mu opioid receptors in the vascular tissue results in vasodilation]
Which of the following opioid antagonist is orally effective?
a) Buprenorphine
b) Naltrexone
c) Naloxone
d) Fentanyl
b
[you should be able to differentiate between naloxone and naltrexone; naloxone is not effective orally; naloxone is used for opioid intoxication/overdose; while naltrexone is effective orally; naltrexone is used to prevent relapse in detoxified opioid addict; administering naltrexone in an opioid-dependent patients can precipitate withdrawal symptoms; Buprenorphine is a partial agonist that is used for both the treatment of pain and the treatment of patients undergoing opioid withdrawal; Fentanyl is potent opioid agonist that is extensively abused]
Opioids are also indicated in which of the following conditions?
a) Pupillary constriction
b) Chronic obstructive pulmonary disease
c) Atrial fibrillation
d) Diarrhea
d
[Remember opioids cause constipation; so this side effect can be used to treat diarrhea or increase in gastric motility; e.g. loperamide; Opioid agonist produce pupillary constriction; Opioid agonist are either contraindicated or should be used with great caution in patients with chronic obstructive pulmonary disease; Opioids have no role in atrial fibrillation]
AM, a 20 year old boy, is brought to the emergency room by his friend because he has stopped breathing. His history shows that he was prescribed an opioid for his postsurgical pain for 30 days last week. On examination he has pinpoint pupils, bradycardia and cold extremities. Based on his signs and symptoms, what do you think is the diagnosis of this patient?
a) Lung collapse
b) Drug interaction
c) Cardiac failure
d) Opioid overdose
d
[remember signs and symptoms of opioid overdose such pinpoint pupils, bradycardia, decreased respiratory rate, cold clammy feet]
In question 21 above, which of the following opioid drugs do you think AM was prescribed?
a) Oxycodone
b) Naltrexone
c) Ketamine
d) Naloxone
a
[Oxycodone is the only opioid agonist in the options; ketamine is a NMDA receptor antagonist; naltrexone and naloxone are opioid antagonists and cannot cause opioid intoxication]
In question 22 above, how will you treat this patient if you are in the emergency room?
a) Oxymorphone
b) Naloxone
c) Naltrexone
d) Pentazocine
b
[Naloxone is used for treating opioid overdose and/or rapid reversal of opioid intoxication; Naltrexone is an orally administered opioid antagonist and is not used for opioid overdose treatment; In fact naltrexone is used for prevention of relapse in patients withdrawing from opioid addiction]
DM is a 45 year old patient undergoing a major surgical procedure for herniated lumbar discs. Which of the following analgesics will you use to facilitate analgesia during the surgical procedure?
a) Naltrexone
b) Fentanyl
c) Oxycodone
d) Oxymorphone
b
t[Fentanyl is a highly potent opioid agonist that can be administered intravenously and has fast onset of action and hence it is sometimes used as anesthesia for surgical procedures; Oxycodone and oxymorphone are pain killers used to relive pain after surgical procedures; Naltrexone is an orally administered opioid antagonist that is used for treatment of relapse in withdrawing opioid dependent patients]
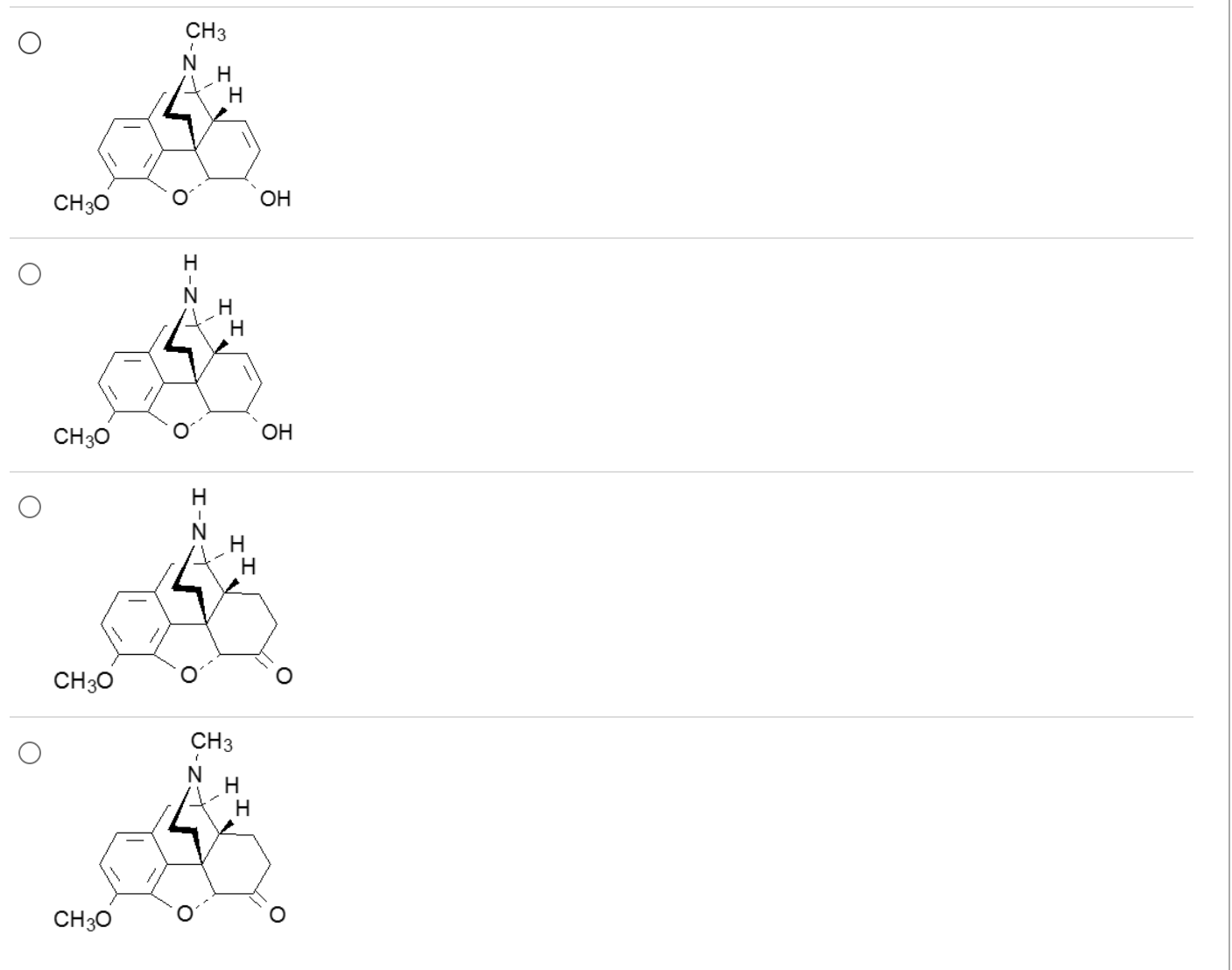
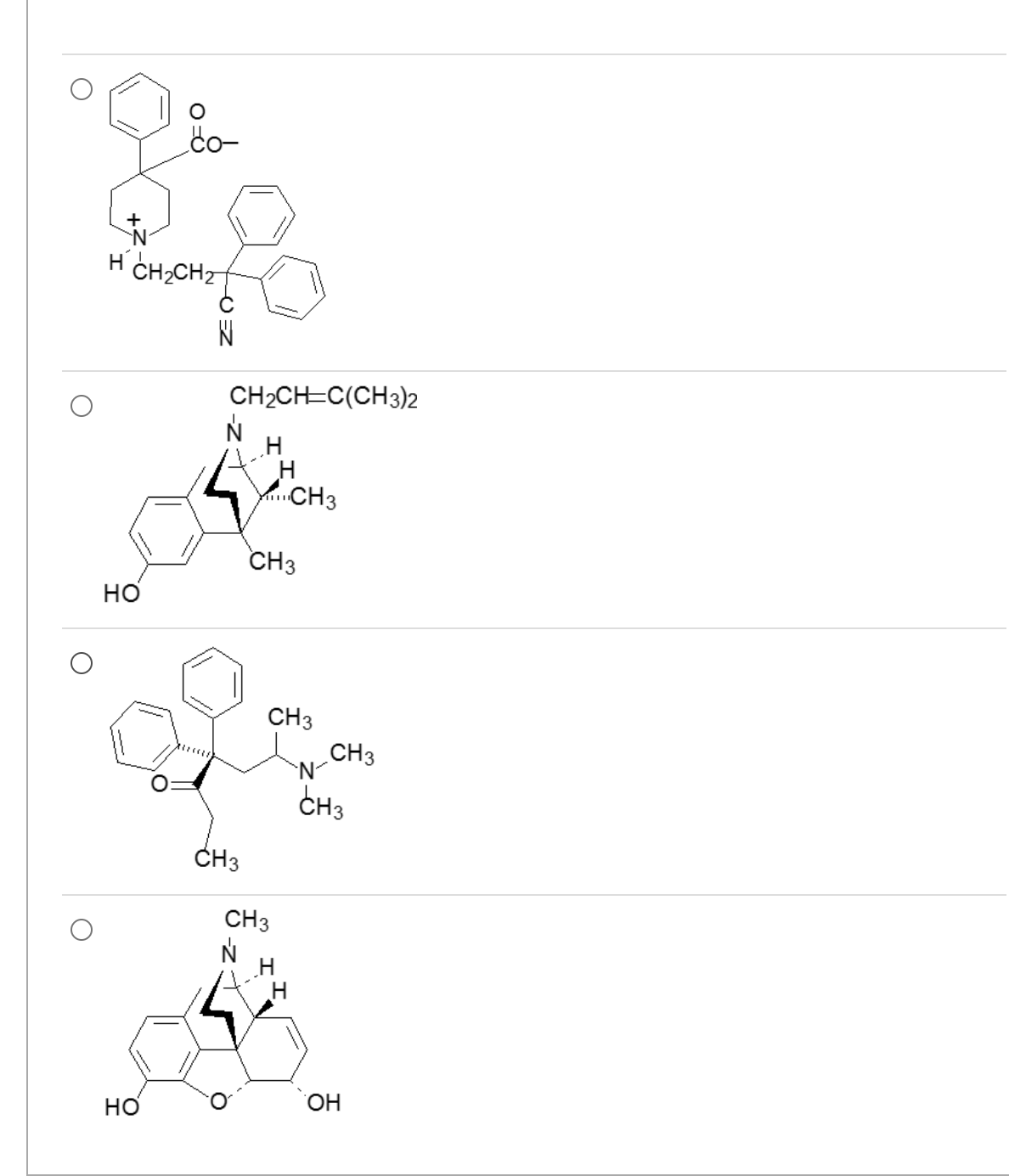
Which one is the most potent mu agonist?
4
all molecules are 3-methyl ethers
3o amines >>>> 2o amines
6 C=O > 6-hydroxyl
7,8 C-C > 7,8 C=C
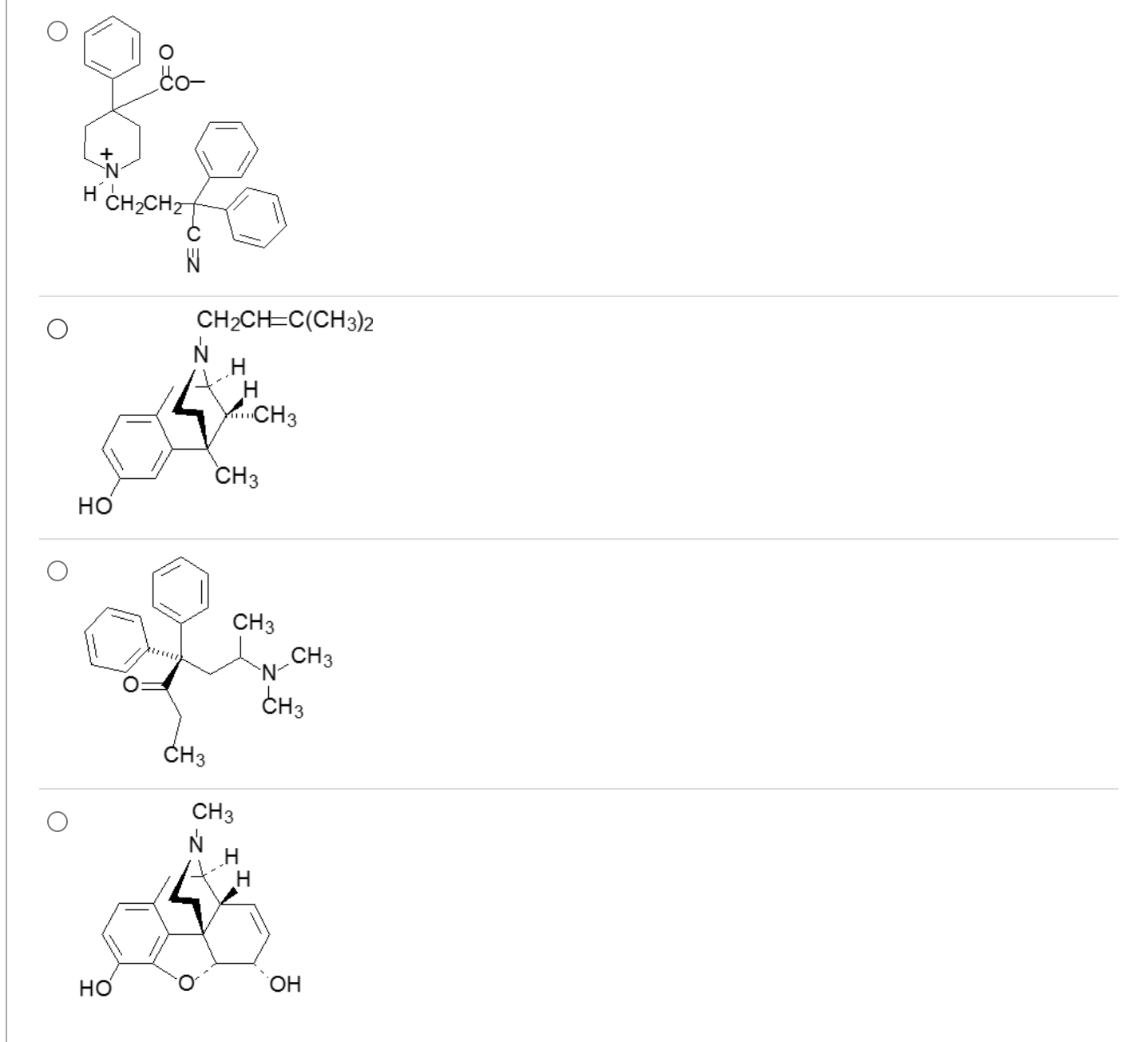
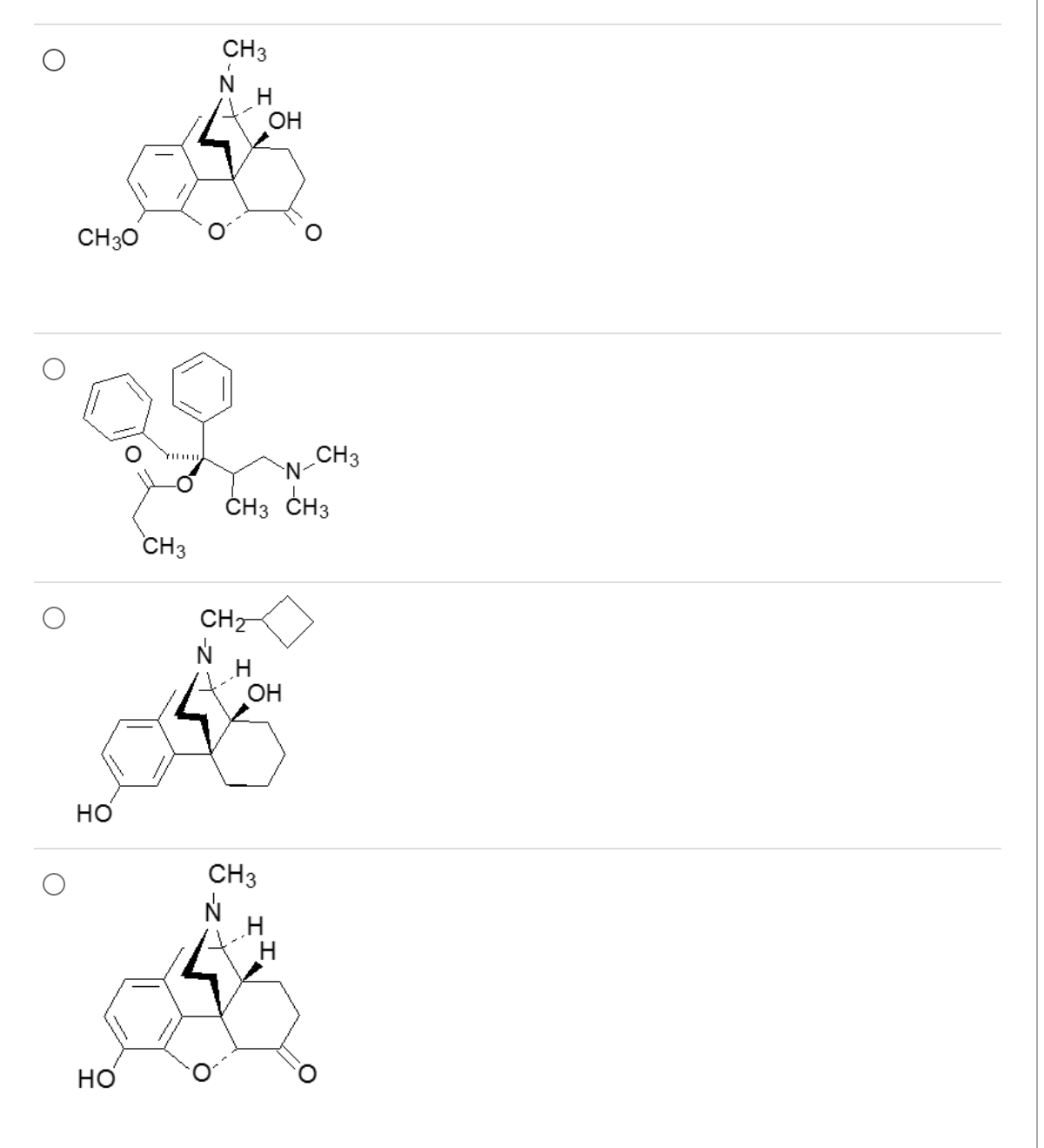
Which one of these demonstrates antidiarrheal effects with minimal to no CNS activity?
1
acid, and 3o amine will be ionized producing a zwitterion (limited ability to cross BBB and accumulate in CNS)
fusion of meperidine and methadone fragments limits central analgesic properties, but permits peripheral actions that decrease GI motility (constipating agent that can be used to treat diarrhea)
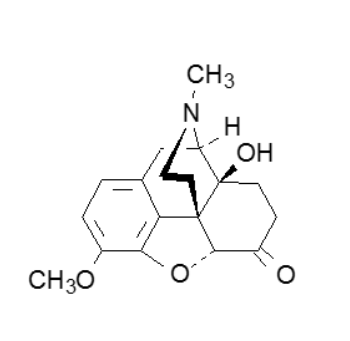
The structural feature of this drug that greatly reduces antitussive properties is
7,8-single bond
6-ketone
14-hydroxyl
3-methyl ether
tertiary amine
3
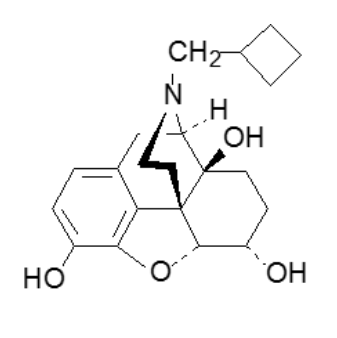
What term best describes this drug?
endorphin
neurohormone
full agonist
full antagonist
mixed agonist-antagonist
5
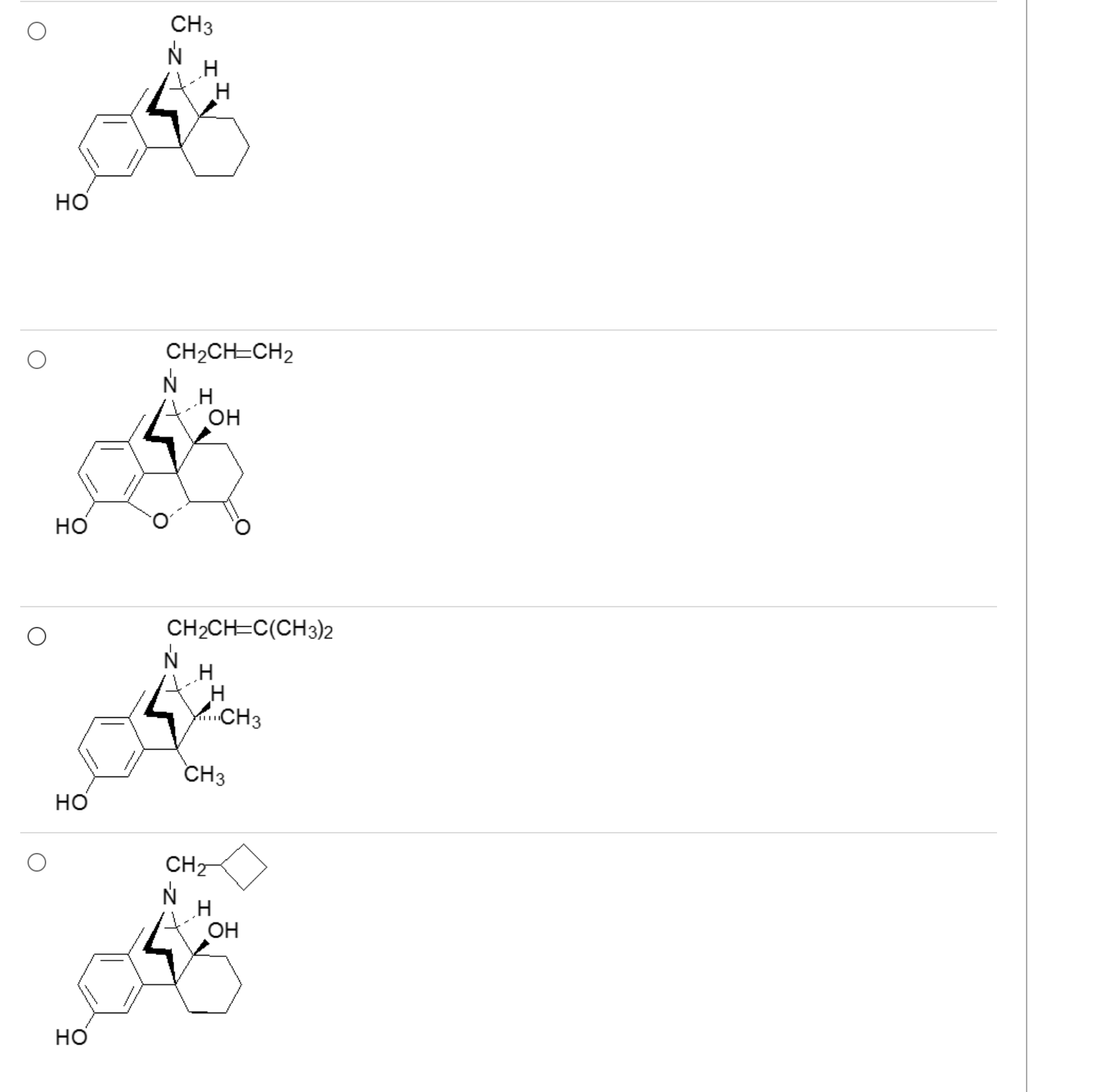
What drug is a mu, kappa, and delta antagonsist?
2
opiates with a 3o allyl amine are antagonists
3-4 carbons with good double bond character = antagonist (unless special case for C ring modifications)
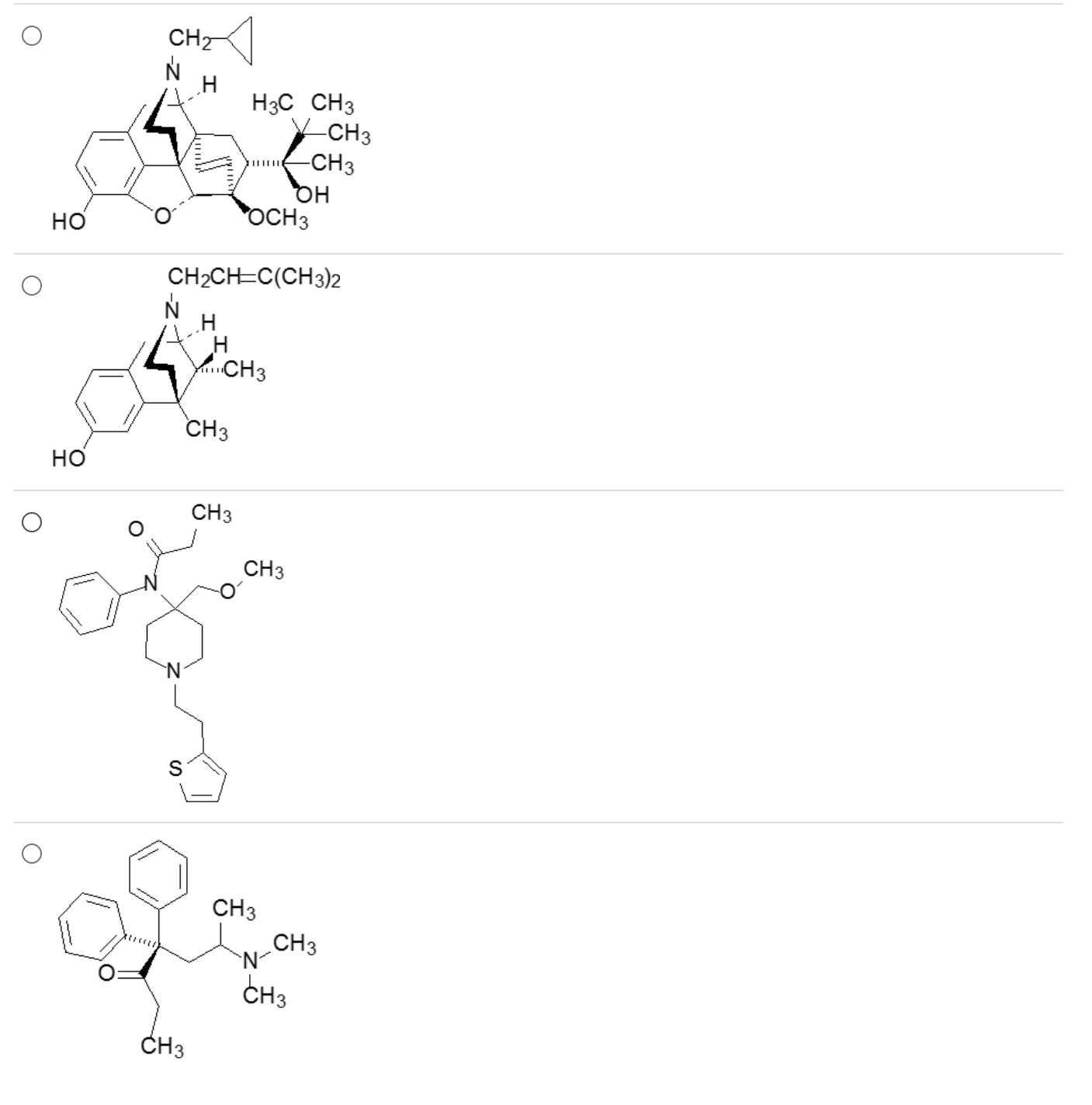
Which of these is an analgesic that’s a member of the methadone class?
4
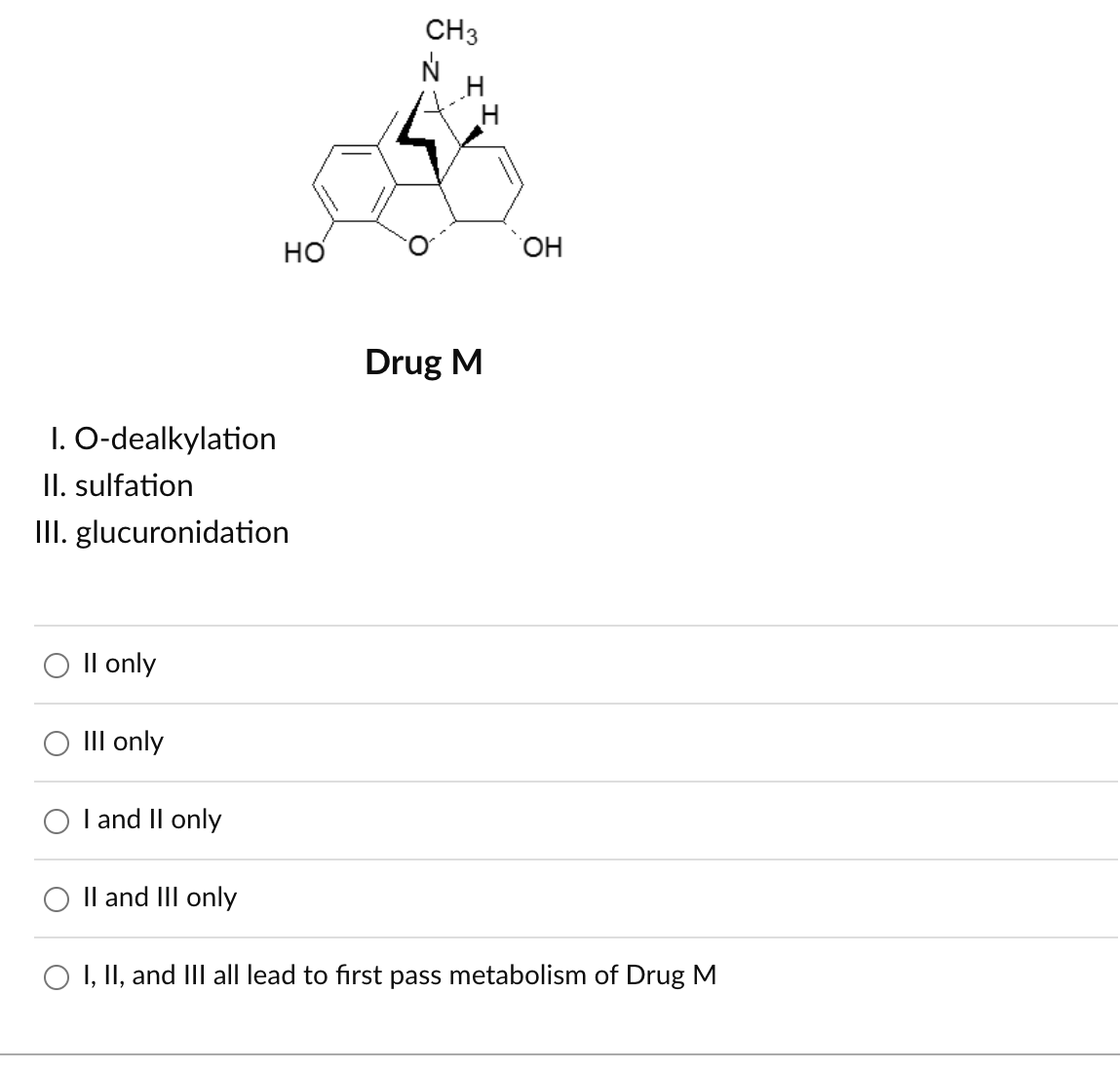
First pass metabolic transformations that reduce the total amount of orally administered Drug M available to penetrate the BBB include:
II and III only
The termination of [Leu5]-enkephalin by a carboxypeptidase is demonstrated by
H-Tyr-GlyOH + H-Gly-Phe-Leu-OH
H-Tyr-Gly-Gly-H + H-Phe-Leu-OH
H-Tyr-OH + H-Gly-Gly-Phe-Leu-OH
H-Tyr-Gly-Gly-Phe-OH +H-Leu-OH
4
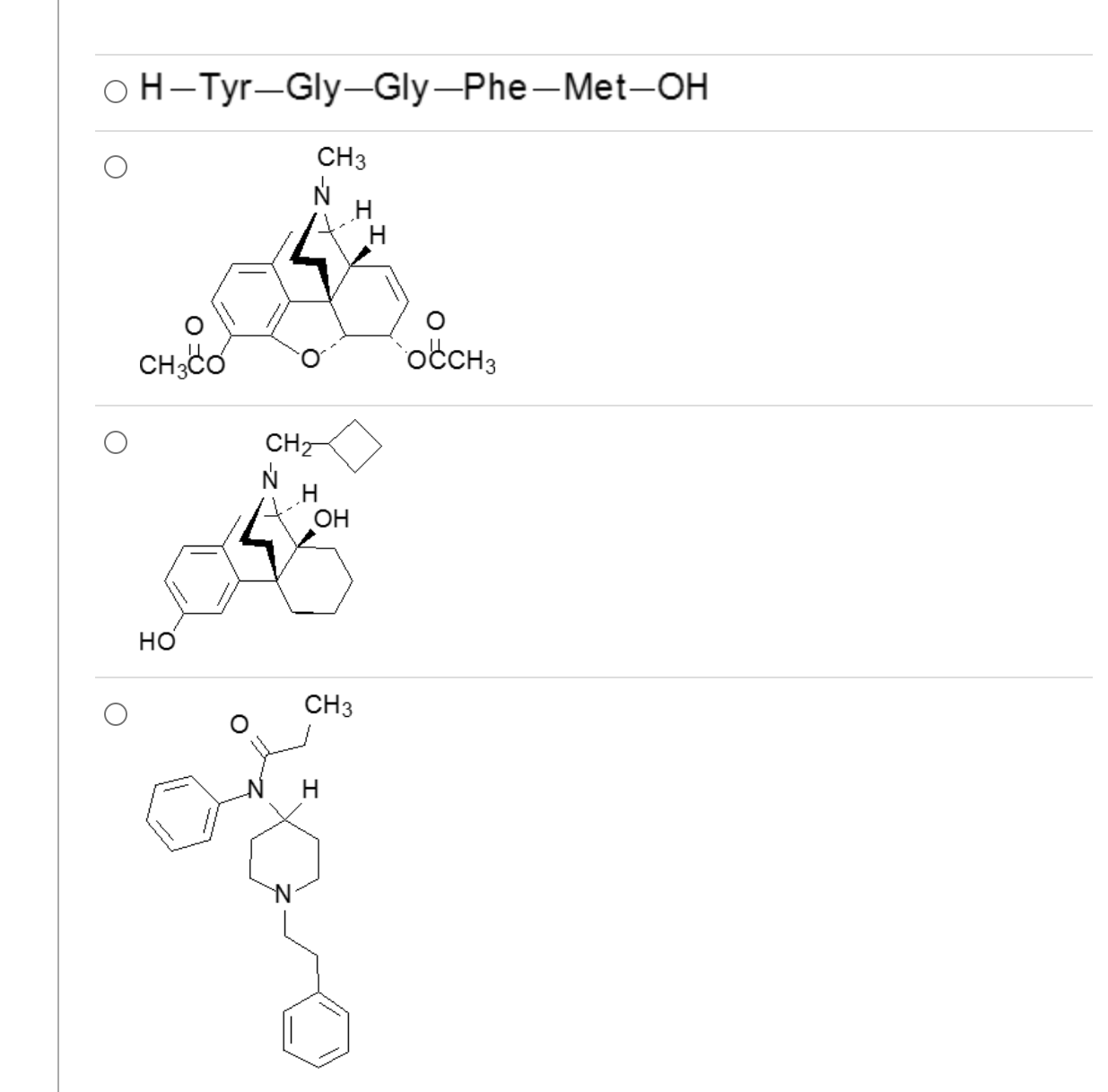
Which one of these is a mixed agonist-antagonist?
3
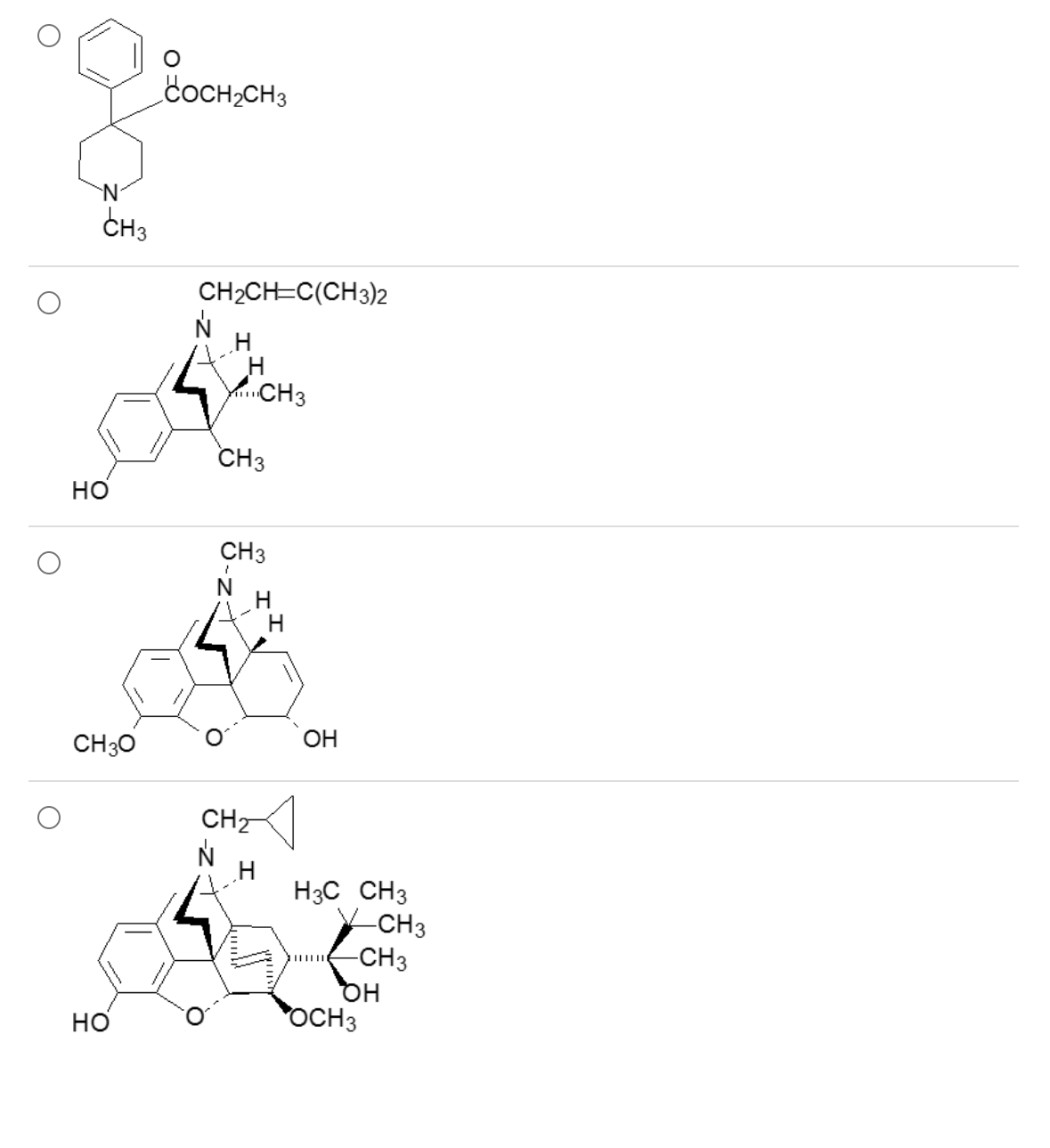
Which one of these is an antidiarrheal agent with negligible CNS analgesic effects?
1
Poor CYP2D6 metabolizers would have a poor or inadequate response to pain relief provided by which of these?
4
If N-delakylation occurs via the CYP2D6 isozyme, an inactive 2o amine would be produced.
Therefore, a poor metabolizer would accumulate the more active parent molecule, and would have enhanced therapeutic effects.

?
I and II only
Arg and Lys residues have basic functional groups on their side chains. Cleavage would result in the release of Leu5-enkephalin.
I. H-Tyr-Gly-Gly-Phe-Leu-Arg-Lys-Tyr-Pro-Lys-OH
II. H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-OH
Positions 6 & 7 of III are occupied by Thr & Ser, which have hydoxyl groups on aliphatic side chains. Peptide III will not be cleaved by dibasic processing enzymes.
III. H-Tyr-Gly-Gly-Phe-Met-Thr-Ser-Glu-Lys-Ser-Gln-Thr-Pro-Leu-Val-Thr-Leu-Phe-Lys-Asn-Ala-Ile-Ile-Lys-Asn-Ala-Tyr-Lys-Lys-Gly-Glu-OH
A mutant species of the opioid poppy has been engineered to produce a precursor necessary for the semi-synthetic production of which of these?
4
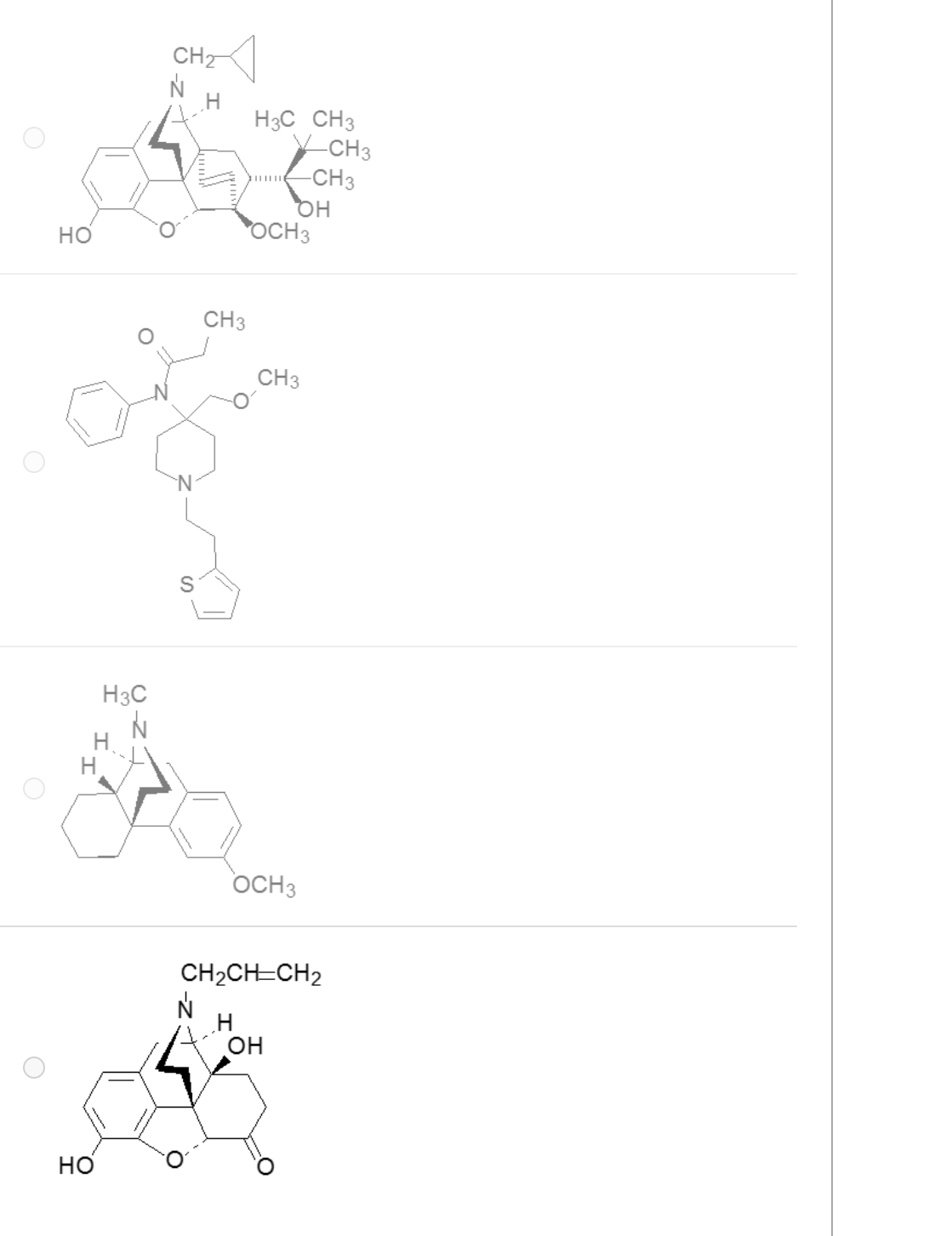
The antagonist of all opioid receptor subtypes is which of these drugs?
4
The neurotransmitter life cycle process that demonstrates [Leu5]-enkephalin has classical neurotransmitter characteristics is
that biosynthesis occurs via the step by step conjugation of carbohydrate building blocks
a selective activation of omega receptor subtypes
synaptic activity is terminated by ORT (opioid reuptake transporter)
synaptic activity is terminated by enkephalinase
4
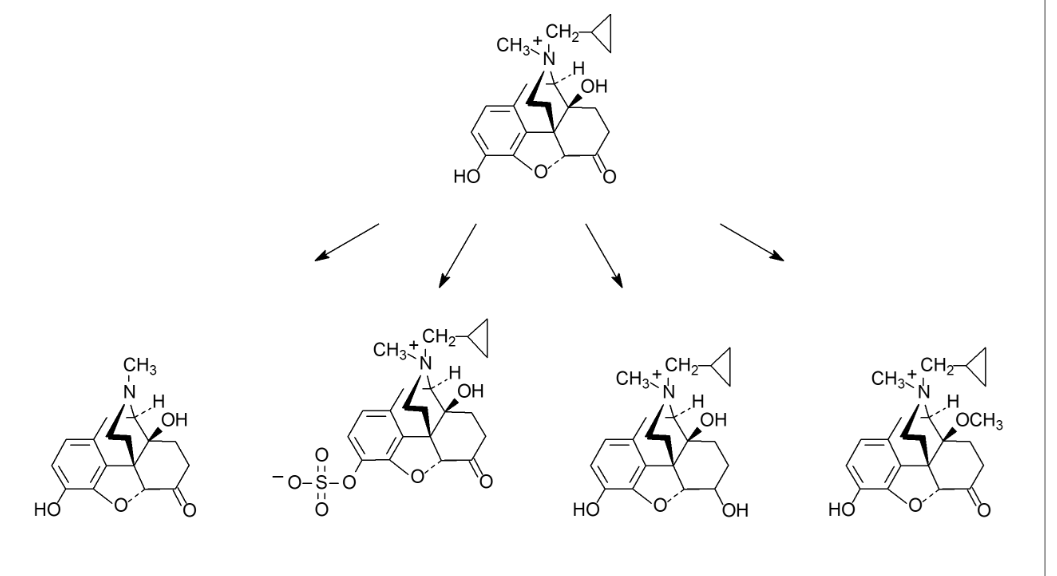
If taken orally, which of the following first pass metabolic transformations produces an active metabolite that is able to penetrate the BBB?
1
Select all that apply. Which of the following pain types is treated by methadone?
Somatic pain
Neuropathic pain
Visceral pain
Musculoskeletal pain
1,2
James was recently started on an opioid medication: Hydrocodone/Acetaminophen 10/325, Take 1-2 tablets PO Q4-6hr PRN. Since his pain has been severe, he has been taking 2 tablets every 4 hours while he is awake (total of 8 tablets/day). Since starting this medication, James has been reporting that he has been seeing things that do not exist, such as spiders crawling on the wall when there are none there. James' experience is most likely related to a/an:
A side effect, wait for tolerance to develop
Peak-dose of opioid
Allergic reaction
Provide prochlorperazine 10 mg PO q6h PRN
2
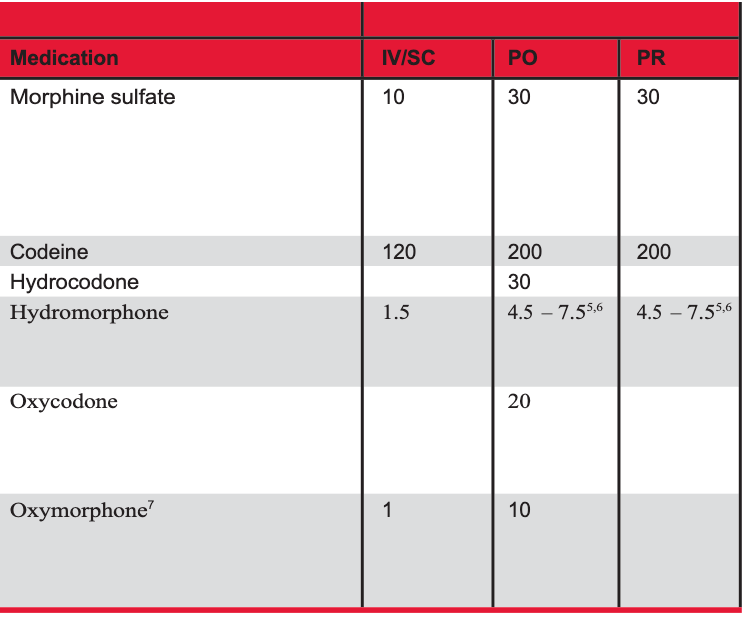
Which of the following scenarios would indicate opioid tolerance?
Oxycodone IR 5mg PO q6h ATC x 3 weeks
Hydrocodone/APAP 7.5/325 take 2 tab PO QID x 7 days
Morphine IR 10 mg PO q6h ATC x 2 weeks
Hydrocodone/APAP 7.5/325 take 2 tab PO QID x 2 weeks
4
Tolerance is >60 MME for >1 week
Janice is taking morphine ATC and reports constipation. Which of the following options would best address this issue?
Exercise
Dietary fiber
Schedule a laxative
Decrease morphine dose
3
Select all that apply. Which of the following are signs and symptoms of opioid withdrawal?
Respiratory depression
Anxiety
Bradycardia
Constipation
Diarrhea
Tachycardia
Sweating
Bradypnea
Insomnia
2,5,6,7,9
Janice presents with stabbing, shooting pain down the back of both thighs. She presents today for a medication recommendation for the treatment of her pain. Which of the following would be the best choice for her situation?
Morphine IR 5 mg PO q4-6h PRN
Tramadol 50 mg PO TID
Nortriptyline 10 mg PO qHS
Lidoderm patches TD Apply topically for 12 hours then remove
3
Janice has stabbing, shooting pain down the back of both thighs that she rates at 7/10. She was started on gabapentin 300mg PO QHS and has slowly been titrated to a dose of gabapentin 600mg po Q8hr, which she reports tolerating without side-effects. She is still reporting pain, however it is slightly improved at 5/10, but her goal is 3/10. When further titrations were attempted (> 600mg Q8hr) she reported sedation and some mild confusion. Which of the following strategies would be best for her situation?
Add venlafaxine 37.5 mg PO qD
Add hydrocodone/APAP 5/325 take 1 tab PO q4-6h PRN
Add lidoderm patches TD apply for 12 hours then remove
Add venlafaxine 75 mg PO qD
Add tramadol 50 mg PO q6h PRN
1
When should naloxone be offered to a patient receiving treatment with opioids?
Taking opioids around the clock (ATC)
Concurrent use of antidepressants
History of depression
30-40 OME/MME
History of substance abuse disorder
5
Pete has a lower back injury following a motor vehicle accident. He describes his pain as dull, aching, throbbing, non-diffuse pain in his lower back region with some radiating, burning pain down the back of his right leg. Which of the following option(s) best matches his pain description?
Neuropathic and visceral pain
Somatic and neuropathic pain
Visceral and somatic pain
Arthritis pain
2
Please select the breakthrough/PRN dose for order: Oxycodone IR 20mg PO Q4hr ATC.
Oxycodone IR 15mg PO Q4-6hr prn
Oxycodone IR 10mg PO Q4-6hr prn
Oxycodone ER 10mg PO Q4-6hr PRN
Hydrocodone/APAP 7.5mg Q4-6hr PRN
2
What are the indications of phenytoin and fosphenytoin?
Seizures, status, trigeminal neuralgia
What formulations of phenytoin and fosphenytoin are available?
phenytoin is available as a capsule, IV solution, suspension, and chewable tablet; fosphenytoin is only available as a solution for IV and IM injection
What does phenytoin equivalent (PE) mean?
Way to equate fosphenytoin to phenytoin since phosphate adds weight
Phenytoin salt factor for injection, capsule, and fosphenytoin
92%
What is fosphenytoin compared to phenytoin?
Prodrug to phenytoin
Phenytoin salt factor for chewable tablets and suspension
1
What is the rate of oral absorption for phenytoin?
non-linear, dose dependent
What is the maximum oral one-time dose of phenytoin?
400 mg
How do you calculate volume of distribution (V) for phenytoin dosing?
0.65 L/kg * weight
What weight do we use to calculate volume of distribution in obese patients?
Adjusted body weight
Adjusted body weight equation for phosphenytoin
IBW + 1.3(ABW-IBW)
What factors will affect phenytoin binding in plasma?
hypoalbuminemia, ESRD
How is phenytoin primarily cleared?
hepatic (CYP2C9, CYP2C19)
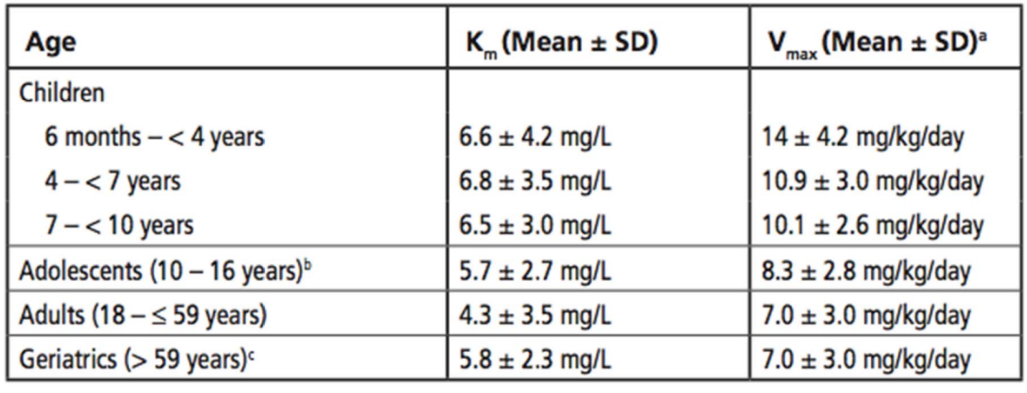
What is the average Vmax (mg/day) of an 11 year old female pt (weight=80 lbs, height=4 ft)
8.3 mg/kg/day * 80 lbs/ 2.2lbs/kg = 301.8 mg/day
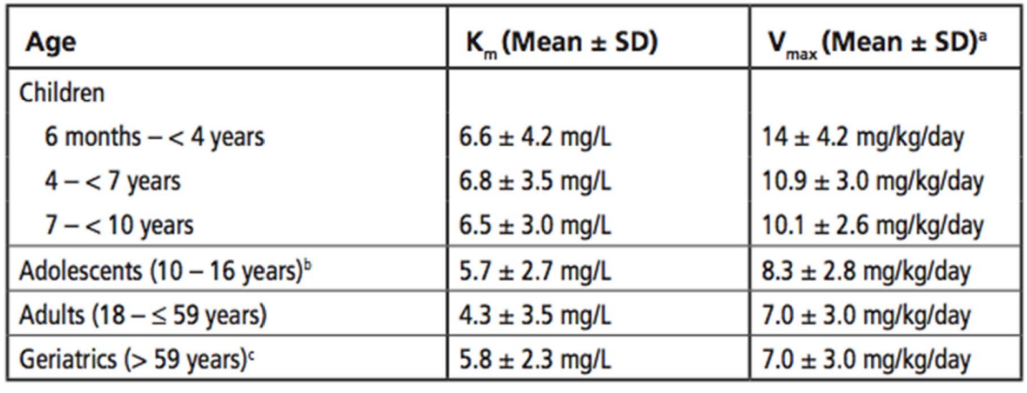
What is the average Vmax (mg/day) of a 30 year old male pt (weight=253 lbs, height=5’11”)
IBW= 2.3(11) +50= 75.3 kg
ABW= 253/2.2= 115 kg
115/75.3= 1.53 → pt is obese
7.0 mg/kg/day * 75.3 kg= 527.1 mg/day
What is the maximum infusion rate of phenytoin?
50 mg/min
What is the maximum infusion rate of fosphenytoin?
150 mg PE/min
What effect does phenytoin have on CYP450 enzymes?
Enzyme inducer
What type of pharmacokinetics does phenytoin display?
non linear (MIchaelis-Menten)
What is the therapeutic plasma concentration of total drug of phenytoin?
10-20 mg/L
When do we measure phenytoin concentration after loading dose?
IV: 2 hrs after infusion, IM: 4 hours after injection, PO: 24 hours after dose
When do we measure phenytoin concentration after maintenance dose?
within 3-4 days, at steady state q7-14 days