Chemistry Exam (I may need to remember)
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
How does NMR work?
Uses radio waves
change spin states of protons and neutrons
How does IR spec work?
Absorbs infrared radiation
changes vibrational energy states of covalent bonds in molecules
How does mass spec work?
molecule is vaporised (gaseous
Bombarded with electrons to ionise it
How does HPLC work?
Equation for mass spec?
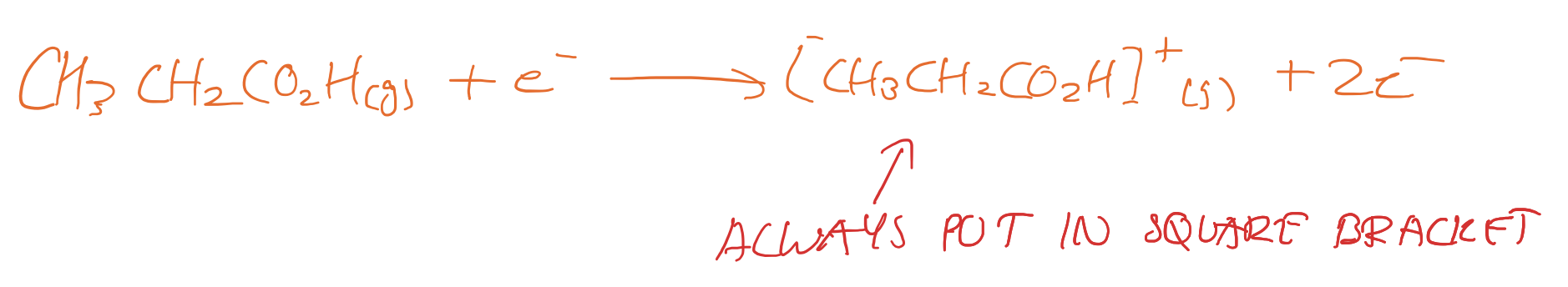
Upfield/downfield is which way?
What does it show?
What is shielding?
Upfield is more shielded (closer to TMS/0/right)
connected or closer to electronegative atom (PULLS electrons to itself)
Can galvanic cells have porous electrodes?
YES! Fuel cells and galvanic cells can both have porous electrodes.
they increase the surface area for the reaction
What is the purpose of a electrolyte in a fuel cell?
What is the purpose of a porous electrode in a fuel cell?
Primary structure of a protein?
The specific sequence of amino acids in the protein chain.
simplest level of a protein structure
shows peptide links only
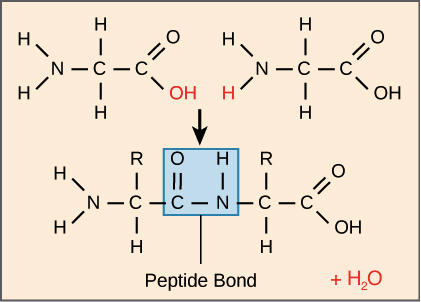
Secondary structure of a protein?
Chains coiling or pleating due to the formation of H-bonds between peptide links
hydrogen bonds, polar interactions are shown
Tertiary structure of a protein?
Overall 3D shape
Stabilised by hydrogen bonds, dipole-dipole, dispersion, ionic interactions, covalent crosslinks (disulphide links S-S)
Quaternary structure of a protein?
2 or more polypeptide links (10 or more amino acids)
hydrophilic and hydrophobic interactions
What does denaturation do?
changes the active site (shape) of the enzyme
can alter the secondary, tertiary and quaternary (if possible)
is irreversible
Optical isomers?
OR enantimers
same molecular formula
same semi-structural formula
different spatial arrangement
Chiral
can’t be superimposed
—> left foot can’t fit in right shoe
Achiral
can be superimposed
—> water bottle
in a scientific poster, which section would explain the reason for undertaking an investigation?
intro
Hydrogen bonging takes place during the formation of__
secondary, tertiary and quaternary structures only. NO PRIMARY
Cellular respiration

is an EXOthermic reaction (negative delta H)
Glucose is oxidised and loses electrons
IN AQEOUS
petrodiesel and biodiesel
both derived from plant and animals
Biodisel —> crops and whatnot
petroldiesel —> formed over millions of years from plants and animals and a ton of pressure and heat
biodiesel is more hygroscopic (attracted to water, likes it) bc of the ester functional group
biodiesel forms crystals at a higher temperature
around the same temp that it boils at is where it’ll form crystals
stronger bonds = boiling point higher but also comes together much easier
Independent variable
The thing we change
on the x-axis
Dependent variable
The thing we measure (according to the aim) (e.g temp change of the water but, we’re trying to find out the delta H)
on the y-axis
Accuracy
How close a result is to the true value (The value that would be found if the quantity could be measured perfectly)
Precision
How close repeated results are to each other
Resolution
How small of a unit of measurement it has
If a syringe has more lines indicating the amount of liquid, then it has a higher resolution
Repeatability
repeated by the same student
similar results and under the same conditions
Reproduceability
performed by different people
produces similar results, under similar conditions
Validity
How well does the experiment address the aim of the experiment
Personal errors
Miscalculations and mistakes (e.g forgetting to do something, reading it wrong, measuring the wrong thing)
Random errors
it’s a random error (e.g needle fluctuates unpredictably between two heights, so don’t know)
Systematic errors
Usually biases one direction (higher or lower)
Cause readings to differ from the true value in a systematic manner so that when a particular value is measured repeatedly, the error is the same.
unit of measurement for concentration
mol/L also known as M
Glycerol formula
C3H8O3
IR Spec
1) hydroxyl
2) carboxyl
3) primary amine
4) secondary amine/alkyne
1) smooth, clean, broad-ish tongue
2) broad, hairy beard
3) vampire fangs
4) single fang
coal seam gas vs coal
coal seam gas releases more energy per gram than coal.
coal seam gas’ major component is CH4
hydrophobic
repels water, doesn’t like it
hydrophillic
likes water, can be dissolved in it
hygroscopic
The ability to absorb water (moisture) from the air.