CH 8: Substitution and Elimination Reactions of Alkyl Halides
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
69 Terms
What makes a good leaving group?
1) weak conjugate base of strong acid
3) weaker base than the nucleophile/base
If you have a bad leaving good, what can you do?
convert it into their conjugate acid through addition of a strong acid (or Lewis acid)
What reaction mechanism does polar aprotic solvents favor?
SN2
alkyl halides or haloalkane
R3 — X (an alkane connected to a halogen)
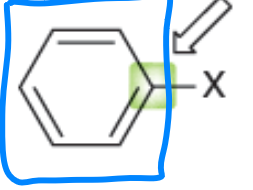
aryl halides
an aromatic ring connected to a halogen
vinyl halides
an alkene connected to a halogen

for alkyl halides, the bond length _________ as a ______ atomic mass atom is attached to the molecule.
increases, higher
for alkyl halides, the most polar molecule is attached to a ______ electronegative atom.
higher
Radical Chain Mechanism (3)
Initiation, Propagation, and Termination
Initiation
1) With some light or heat, X2 splits homolytically and produces 2 radicals (atoms or molecules w/ unpaired e-).
What happens in the 1st propagation step?
Halogen radical bonds with one of H atom’s e- (of the reactant).
What happens in the 2nd propagation step?
In the 2nd step, another X2 homolytically splits again. A halogen radical bonds with the reactant radical.
Termination
Three products form.
Two X radicals bond and molecular halogen forms.
Halogen radical bonds with reactant radical. Haloalkane forms.
Reactant radical bonds with another reactant radical and a new product forms.
Of the three steps of radical chain mechanism, the slowest or rate-determining step is….
propagation
In order to get monosubstituted chloromethane, one way is…
use a higher concentration of alkane in comparison to chlorine
Who is more selective? Chlorination or bromination?
bromination
What are two reasons that alkyl halides undergo substitution and elimination?
Since the halogen is a e- withdrawing group, it creates a partial positive charge on the alpha carbon, making it more susceptible for nucleophilic attack.
What are the 2 possible substitution mechanism?
SN2 and SN1
What happens in SN2 or bimolecular nucleophilic substitution reaction?
one-step (concerted) mechanism
leaving group leaves
nucleophile attacks
happening at the SAME time.
What happens in SN1 or unimolecular nucleophilic substitution reaction?
multi-step mechanism
leaving group leaves
carbocation forms
nucleophile attacks
Why does the nucleophile attack from the back-side?
1) Front-side is e- dense.
2) Homo of nucleophile can flow to the lumo of the electrophile.
What happens to the chiral carbon of the SN2?
The reaction is stereospecific, so the alpha carbon (reaction site) will undergo an inversion of configuration.
What prevents SN2 from happening? What does that mean?
Too much steric hinderince meaning more branching on the alpha and beta carbons of alkyl groups result in a lower rate of rxn.
What determines or influences the strength of the nucleophile?
1) charge
2) resonance
3) electronegativity
4) atomic mass
5) steric hinderance
What makes a strong nucleophile?
1) anion
2) no resonance
3) low electronegativity
4) bigger atomic mass
5) less hindered
What is the PT pattern for a strong nucleophilicity?
Right to Left and Top to Bottom
What are the 8 strong nucleophiles?
chloride ion (Cl-), bromide ion (Br-), iodide ion (I-), HS-, RS-, HO-, RO-, and N(triple bonded)C-
What are the 4 polar aprotic solvent?
DMF (O(double bonded)C(bonded to)H and N(bonded to) R1 and R2, DMS (sulfide), DMSO (O(double bonded)S(bonded to)R1 and R2, and acetone (ketone)
How can you identify a protic solvent?
1) solvent that can donate protons
2) Does it have a OH or NH?
What is the requirements of a SN2 reaction?
strong nucleophile and polar aprotic solvent
What is the rate-determining step in a SN1 reaction?
carbocation intermediate
What is the relationship btwn the carbocation intermediate and the rate of SN1?
directly proportional, so the more stable the carbocation intermediate, the faster the reaction
What is the first question you should ask about the rxn?
The func. (base or nucleophile/strong or weak/less bulky or bulky) of the reagant
What is the second question you should ask about the rxn?
The type of substrate (1°, 2°, or 3°) and the expected mechanism (SN1, SN2, E1 or E2)
What is the third question you should ask about the rxn?
The regiochemical and stereochemical requirements (stereocenter inversion, racemization, antiperiplanar, Zaitsev, and Hoffman)
Starting with the 1st question, what kind of reagants promote SN1, SN2, E1 and E2?
SN1: weak nucleophile
SN2: strong nucleophile
E1: weak base
E2: strong base
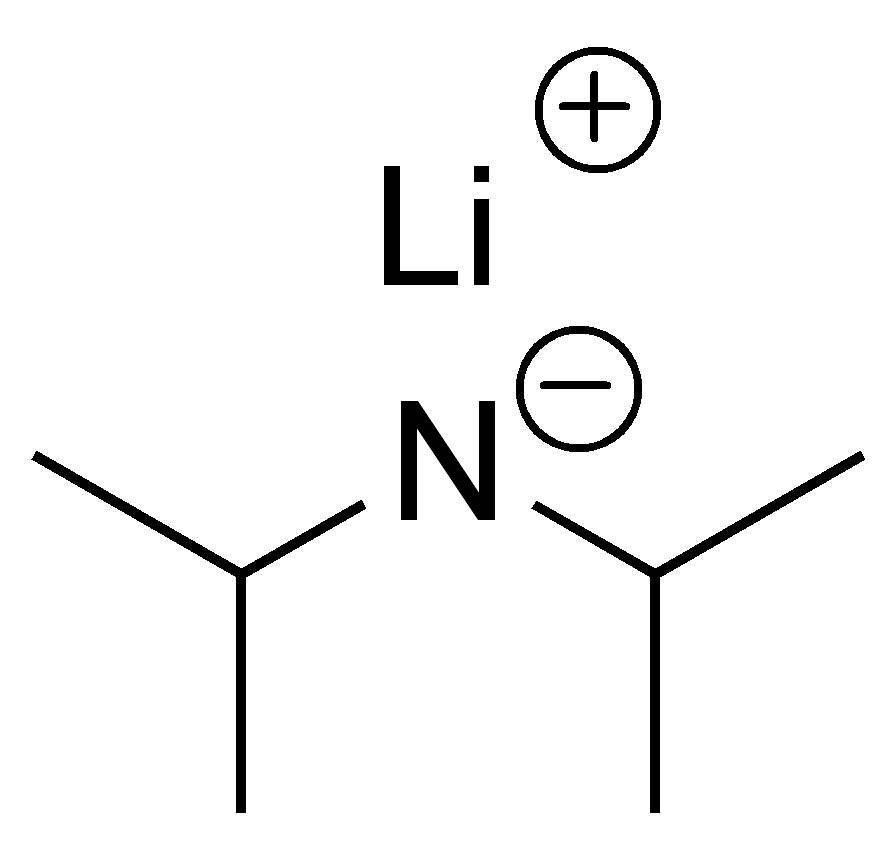
LDA (Lithium Di-isopropyl Amide) - type of B and Nu
SB/WNu
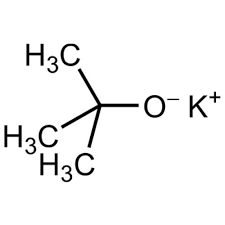
t-buOK - type of B and Nu
SB/wNu
HO-/-OH - type of B and Nu
SB/SNu
MeO-/-OMe/H3CO-/-OCH3 - type of B and Nu
SB/SNu
EtO-/-OEt - type of B and Nu
SB/SNu
I-, Cl-, and Br- - type of B and Nu
WB/SNu
Why are I-, Cl-, and Br- weak bases?
they are conj. bases of strong acids
RS- and HS- - type of B and Nu
WB/SNu
RSH and H2S - type of B and Nu
WB/SNu
H2O - type of B and Nu
WB/WNu
MeOH/CH3OH - type of B and Nu
WB/WNu
EtOH/CH3CH2OH/ - type of B and Nu
WB/WNu
What are the (4) common strong bases used in ochem?
RO- (oxygen chain), NH2-, LDA (lithium di-isopropyl amide), and t-BuO-
What are the (13) strong nucleophiles?
I-, Cl-, Br-, HS-, RS-, HO-, RO-, NC-, RCOO-, R3P, R2NH2, NH3, and R2S (RSH)
What are the (3) weak nucleophiles?
H2O, ROH, and F-
SB/WNu and 1° - type of mechanism
E2
SB/SNu and 1° - type of mechanism
SN2
WB/SNu and 1° - type of mechanism
SN2
WB/WNu and 1° - type of mechanism
NR
SB/WNu and 2° - type of mechanism
E2
SB/SNu and 2° - type of mechanism
E2
WB/SNu and 2° - type of mechanism
SN2
WB/WNu and 2° - type of mechanism
SN1 or E1, heat is the determinator
SB/WNu and 3° - type of mechanism
E2
SB/SNu and 3° - type of mechanism
E2
WB/SNu and 3° - type of mechanism
SN1
WB/WNu and 3° - type of mechanism
SN1 or E1, heat is the determinator
SN2 - regioselectivity/stereoselectivity
concerted (one-step) mechanism, so there is a single product and an inversion of configuration at the alpha-carbon
SN1 - regioselectivity/stereoselectivity
a multi-step mechanism, so the most substituted carbocation will form and the chiral carbon will lead to 2 products (R and S)
E2 - regioselectivity/stereoselectivity
- concerted (one-step) mechanism, so the alkenes will form from having beta-hydrogen that is antiperiplanar to the LG.
- bulky bases —> Hoffman product (or less substituted alkene)
- less bulky bases —> Zaitsev product (or most substituted alkene
- trans disubstituted alkene are favored over cis disubstituted alkene
- E trisubstituted alkene are favored over Z trisubstitutted alkene
E1 - regioselectivity/stereoselectivity
- a multi-step mechanism, so the most carbocation will form and the alkenes will form from having beta-hydrogen
- major product —> most substituted alkene
Stereoselective rxn
one major and one minor product formed
stereospecific rxn
only one product formed