3.10 Cis-Trans Isomerism in Alkenes
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
70 Terms
Arises upon the mixing of one s orbital and two p orbitals to form three degenerate sp2 orbitals, leaving behind one non-hybridized p-orbital (in the perpendicular position)
sp2 hybridization
sp2 electronic geometry
trigonal planar (three sp2 orbitals arranged around a central point, positioned to be as far apart as possible, forming a trigonal shape)
The sp2 ideal bond angle is ____
120 degrees
We saw in the chapter on Structure and Bonding that the carbon–carbon double bond can be described in two ways.
→ In ________ _____ theory
→ In ________ _________ theory
Valence bond
Molecular orbital (MO)
In valence bond language, the carbons are sp2-hybridized and have three equivalent hybrid orbitals that lie in a plane at angles of 120° to one another. The carbons form a σ bond by a head-on overlap of sp2 orbitals and form a __ bond by sideways overlap of unhybridized p orbitals oriented perpendicular to the sp2 plane,
π
In ________ _______ language, interaction between the p orbitals leads to one bonding and one antibonding π molecular orbital. The π bonding MO has no node between nuclei and results from a combination of p orbital lobes with the same algebraic sign. The π antibonding MO has a node between nuclei and results from a combination of lobes with different algebraic signs.
molecular orbital
___________ means that two substituents other than hydrogen are bonded to the double-bond carbons
disubstituted
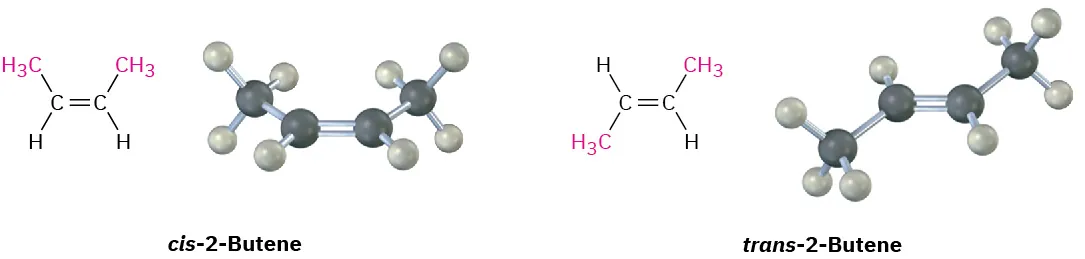
Since bond rotation can’t occur, the two 2-butenes can’t spontaneously interconvert; they are different, isolable compounds. As with disubstituted cycloalkanes, we call such compounds ___-____ __________. The compound with substituents on the same side of the double bond is called cis-2-butene, and the isomer with substituents on opposite sides is trans-2-butene
cis-trans stereoisomers
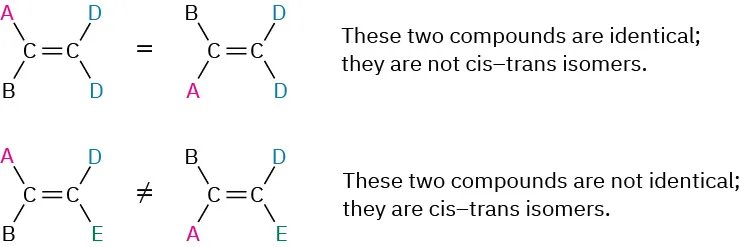
Cis–trans isomerism is not limited to disubstituted alkenes. It can occur whenever both ________-bond carbons are attached to two different groups.
double
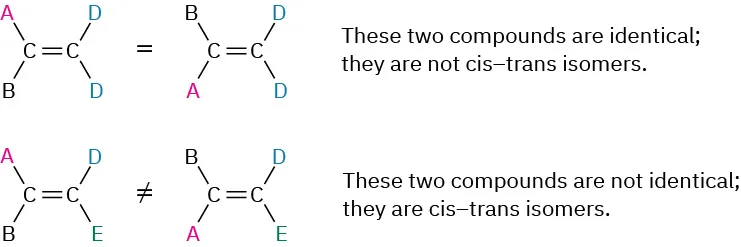
If one of the double-bond carbons is attached to two identical groups, however, cis–trans isomerism is ___ possible
not
(7-8)
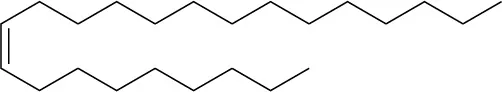
The sex attractant of the common housefly is an alkene named cis-9-tricosene. Draw its structure. (Tricosane is the straight-chain alkane C23H48.)
(7-9)
Which of the following compounds can exist as pairs of cis–trans isomers? Draw each pair, and indicate the geometry of each isomer.
(a) CH3CH=CH2
(b) (CH3)2C=CHCH3
(c) CH3CH2CH=CHCH3
(d) (CH3)2C=C(CH3)CH2CH3
(e) ClCH=CHCl
(f) BrCH=CHCl
Compounds (c), (e), and (f) have cis–trans isomers
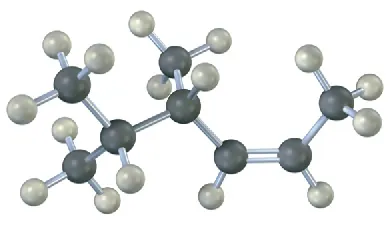
(7-10)
Name the following alkenes, including a cis or trans designation:
(a)
cis-4,5-Dimethyl-2-hexene
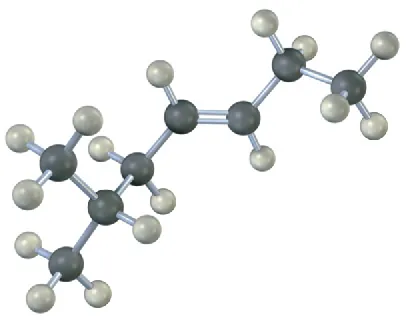
(7-10)
Name the following alkenes, including a cis or trans designation:
(b)
trans-6-Methyl-3-heptene
The cis–trans naming system used in the previous section works only with ___________ alkenes—compounds that have two substituents other than hydrogen on the double bond. With trisubstituted and tetrasubstituted double bonds, a more general method is needed for describing double-bond geometry.
disubstituted
______________ means three substituents other than hydrogen on the double bond; ____________ means four substituents other than hydrogen.)
trisubstituted
tetrasubstituted
The method used for describing alkene stereochemistry is called the _,_ _______
E,Z system
E,Z system
Rule 1:
Considering each of the double-bond carbons separately, look at the two substituents attached and rank them according to the ________ _______ of the first atom in each (8 for oxygen, 6 for carbon, 1 for hydrogen, and so forth). An atom with higher atomic number ranks higher than an atom with lower atomic number.
atomic number
E,Z system
Rule 2:
If a decision can’t be reached by ranking the first atoms in the two substituents, look at the second, third, or fourth atoms away from the double-bond until the first difference is found.
(info card)
E,Z system
Rule 2:
If a decision can’t be reached by ranking the first atoms in the two substituents, look at the second, third, or fourth atoms away from the double-bond until the first difference is found.
(info card)
E,Z system
Rule 3:
Multiple-bonded atoms are equivalent to the same number of single-bonded atoms.
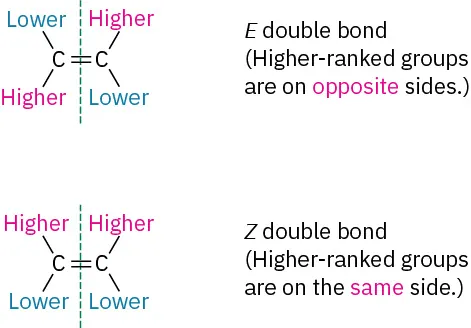
Once the two groups attached to each double-bonded carbon have been ranked as either higher or lower, look at the entire molecule. If the higher-ranked groups on each carbon are on the same side of the double bond, the alkene is said to have a configuration, for the German zusammen, meaning “together.” If the higher-ranked groups are on opposite sides, the alkene has an _ configuration, for the German entgegen, meaning “opposite.” (For a simple way to remember which is which, note that the groups are on “ze zame zide” in the Z isomer.)
Z
E
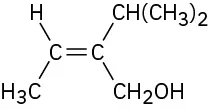
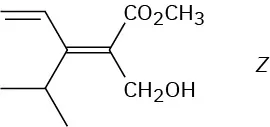
Assign E or Z configuration to the double bond in the following compound:
The left-hand carbon has −H and −CH3 substituents, of which −CH3 ranks higher by sequence rule 1. The right-hand carbon has −CH(CH3)2 and −CH2OH substituents, which are equivalent by rule 1. By rule 2, however, −CH2OH ranks higher than −CH(CH3)2 because the substituent −CH2OH has an oxygen as its highest second atom, but −CH(CH3)2 has a carbon as its highest second atom. The two higher-ranked groups are on the same side of the double bond, so we assign a Z configuration.
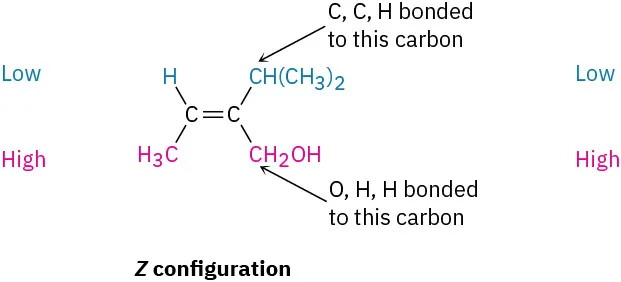
(7-11)
Which member in each of the following sets ranks higher?
(a) −H or −CH3
(b) −Cl or −CH2Cl
(c) –CH2CH2Br or –CH=CH2
(d) −NHCH3 or −OCH3
(e) −CH2OH or −CH=O
(f) −CH2OCH3 or −CH=O
(a) −CH3
(b) −Cl
(c) −CH=CH2
(d) −OCH3
(e) −CH=O
(f) −CH=O
(7-12)
Rank the substituents in each of the following sets according to the sequence rules:
(a)
−CH3, −OH, −H, −Cl
−Cl, −OH, −CH3
(7-12)
Rank the substituents in each of the following sets according to the sequence rules:
(b)
−CH3, −CH2CH3, −CH=CH2, −CH2OH
−CH2OH, −CH=CH2, −CH2CH3, −CH3
(7-12)
Rank the substituents in each of the following sets according to the sequence rules:
(c)
−CO2H, −CH2OH, −C≡N, −CH2NH2
−CO2H, −CH2OH, −C≡N, −CH2NH2
(7-12)
Rank the substituents in each of the following sets according to the sequence rules:
(d)
−CH2CH3, −C≡CH, −C≡N, −CH2OCH3
−CH2OCH3, −C≡N, −C≡CH, −CH2CH3
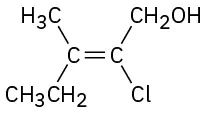
(7-13)
Assign E or Z configuration to the following alkenes:
Z
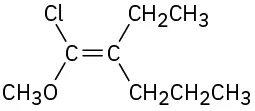
(7-13)
Assign E or Z configuration to the following alkenes:
E
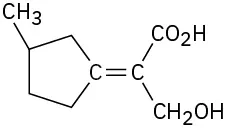
(7-13)
Assign E or Z configuration to the following alkenes:
Z
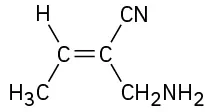
(7-13)
Assign E or Z configuration to the following alkenes:
E
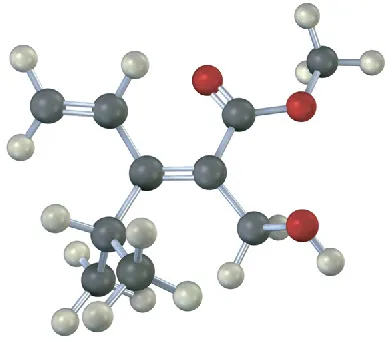
(7-14)
Assign stereochemistry (E or Z) to the double bond in the following compound, and convert the drawing into a skeletal structure (red = O):
Z
“________ _________” is the result of the tetrahedral stereochemistry of sp3-hybridized carbon atom
Molecular handedness
A molecule that is not identical to its mirror image is a kind of stereoisomer called an
enantiomer
Enantiomers are related to each other as a right hand is related to a left hand and result whenever a tetrahedral carbon is bonded to four ________ substituents (one need not be H)
different
when two objects are placed on top of each other, they can be rotated and positioned so that they completely overlap and look identical
superimposable
____________ are two molecules that are mirror images of each other but are not superimposable, and therefore are not identic
enantiomer
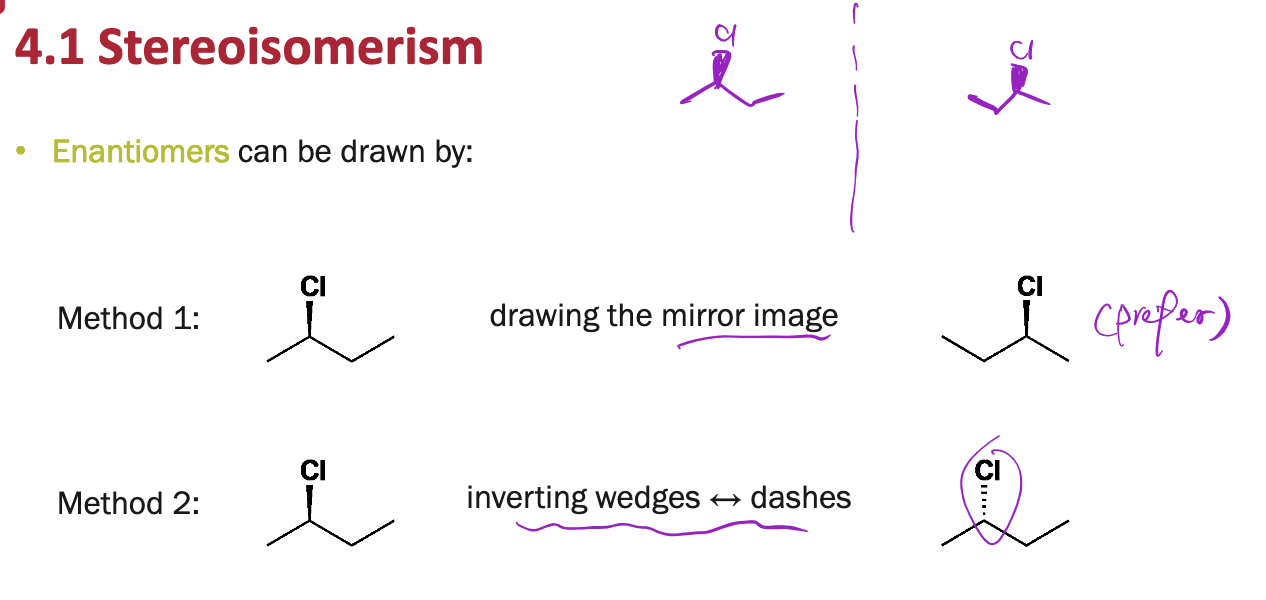
Enantiomers can be drawn in two ways:
(1) ______________
(2) ______________
(1) drawing the mirror image
(2) inverting wedges to dashes (vice versa)
Because enantiomers are mirror images, they will have many similar properties,
but because they are not identical, their pharmacology may be very ________
different
A molecule that is not identical to its mirror image is said to be _______
chiral
a molecule is _____ if it has a plane of symmetry
(A molecule that has a plane of symmetry in any conformation must be identical to its mirror image and must be nonchiral)
achiral
Tetrahedral carbon atoms that are connected to 4 different groups are called
(4 names for this)
chirality centers
stereocenter
asymmetric center
stereogenic center
Note that chirality is a property of the entire molecule, whereas a chirality center is the cause of _______.
chirality
the chirality center (marked with an ______)
asterik
(5-1)
Which of the following objects are chiral?
(a) Soda can
(b) Screwdriver
(c) Screw
(d) Shoe
Chiral: screw, shoe
(5-2)
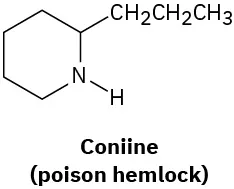
Which of the following molecules are chiral? Identify the chirality center(s) in each.
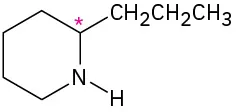
(5-2)
Which of the following molecules are chiral? Identify the chirality center(s) in each.
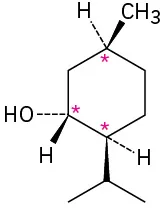
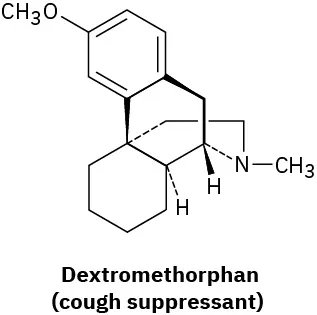
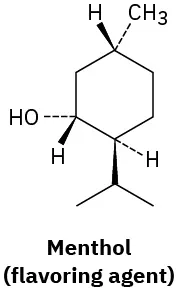
(5-2)
Which of the following molecules are chiral? Identify the chirality center(s) in each.
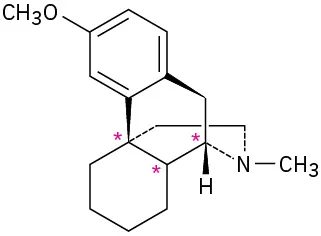
(5-3)
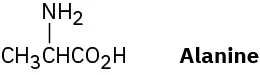
Alanine, an amino acid found in proteins, is chiral. Draw the two enantiomers of alanine using the standard convention of solid, wedged, and dashed lines.
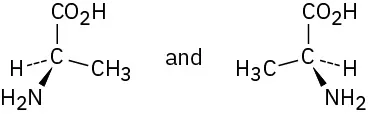
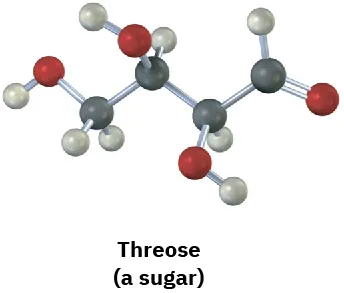
(5-4)
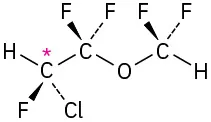
Identify the chirality centers in the following molecules (gray = H, black = C, red = O, green = Cl, yellow-green = F):
(a)
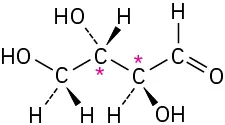
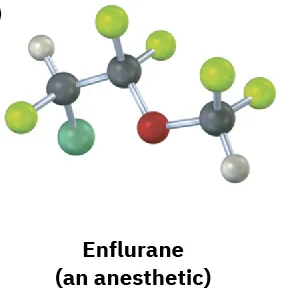
(5-4)
Identify the chirality centers in the following molecules (gray = H, black = C, red = O, green = Cl, yellow-green = F):
(b)
a written method for indicating the three-dimensional arrangement,
configuration
the method used a set of sequence rules to rank the four groups attached to the chirality center and then looks at the handedness with which those groups are attached
Cahn–Ingold–Prelog rules
The Cahn–Ingold–Prelog rules are used to designate each chirality center as having either an _ or _ configuration
R or S
Cahn–Ingold–Prelog:
Rule 1:
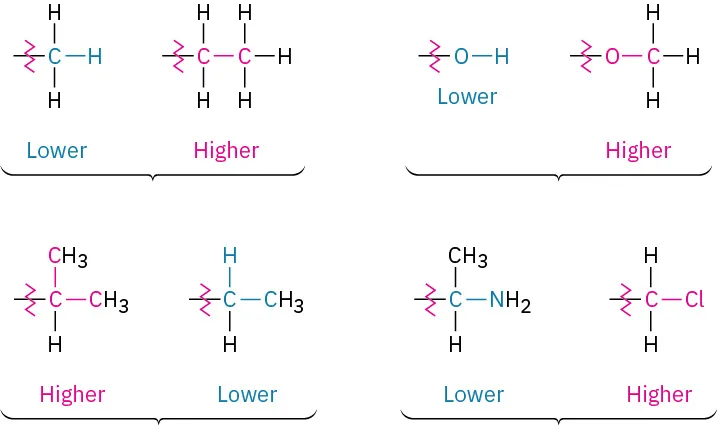
Look at the four atoms directly attached to the chirality center, and rank them according to ______ number.
(The atom with the highest _____ number has the highest ranking)
(When different isotopes of the same element are compared, the heavier isotope ranks higher than the lighter isotope)
atomic
Cahn–Ingold–Prelog:
Rule 2:
If a decision can’t be reached by ranking the first atoms in the substituent, look at the second, third, or fourth atoms away from the chirality center until the first difference is found.
(A −CH2CH3 substituent and a −CH3 substituent are equivalent by rule 1 because both have carbon as the first atom. By rule 2, however, ethyl ranks higher than methyl because ethyl has a carbon as its highest second atom, while methyl has only hydrogen as its second atom.)
(info card)
Cahn–Ingold–Prelog:
Rule 2:
If a decision can’t be reached by ranking the first atoms in the substituent, look at the second, third, or fourth atoms away from the chirality center until the first difference is found.
(A −CH2CH3 substituent and a −CH3 substituent are equivalent by rule 1 because both have carbon as the first atom. By rule 2, however, ethyl ranks higher than methyl because ethyl has a carbon as its highest second atom, while methyl has only hydrogen as its second atom.)
(info card)

Cahn–Ingold–Prelog:
Rule 3:
Multiple-bonded atoms are equivalent to the same number of single-bonded atoms. For example, an aldehyde substituent (–CH═O), which has a carbon atom doubly bonded to one oxygen, is equivalent to a substituent having a carbon atom singly bonded to two oxygens:
(info card)
Cahn–Ingold–Prelog:
Rule 3:
Multiple-bonded atoms are equivalent to the same number of single-bonded atoms. For example, an aldehyde substituent (–CH═O), which has a carbon atom doubly bonded to one oxygen, is equivalent to a substituent having a carbon atom singly bonded to two oxygens:
(info card)
Having ranked the four groups attached to a chiral carbon, we describe the stereochemical configuration around the carbon by orienting the molecule so that the group with the lowest ranking (4) points directly _____ ___us.
away from
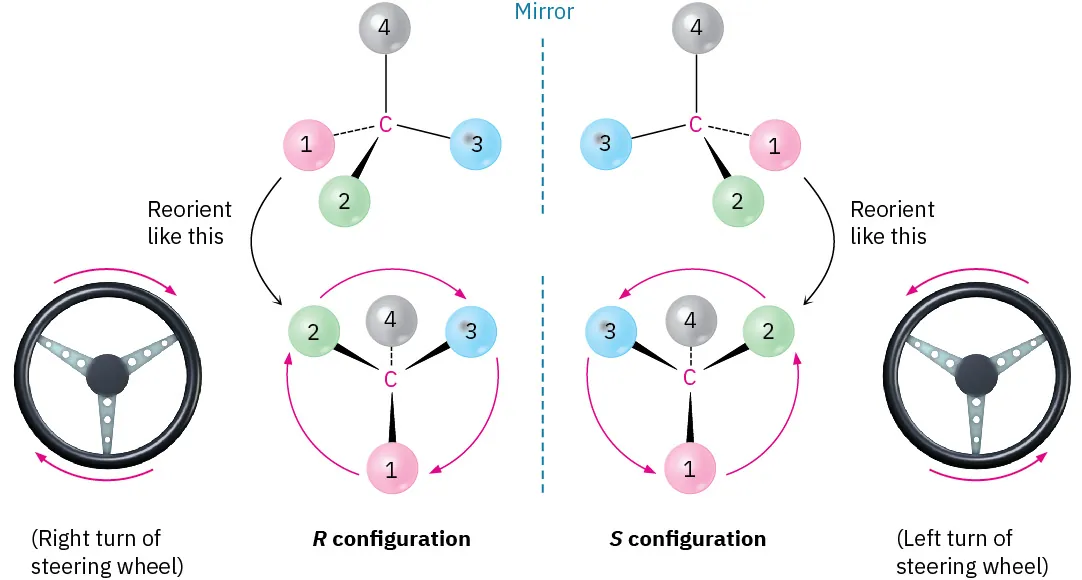
We then look at the three remaining substituents, which now appear to radiate toward us like the spokes on a steering wheel. If a curved arrow drawn from the highest to second-highest to third-highest ranked substituent (1 → 2 → 3) is clockwise, we say that the chirality center has the R configuration (Latin rectus, meaning “right”). If an arrow from 1 → 2 → 3 is counterclockwise, the chirality center has the S configuration (Latin sinister, meaning “left”). To remember these assignments, think of a car’s steering wheel when making a Right (clockwise) turn.
(info card)
We then look at the three remaining substituents, which now appear to radiate toward us like the spokes on a steering wheel. If a curved arrow drawn from the highest to second-highest to third-highest ranked substituent (1 → 2 → 3) is clockwise, we say that the chirality center has the R configuration (Latin rectus, meaning “right”). If an arrow from 1 → 2 → 3 is counterclockwise, the chirality center has the S configuration (Latin sinister, meaning “left”). To remember these assignments, think of a car’s steering wheel when making a Right (clockwise) turn.
(info card)
(5-7)
Which member in each of the following sets ranks higher?
(a) −H or −Br
(b) −Cl or −Br
(c) −CH3 or −CH2CH3
(d) −NH2 or −OH
(e) −CH2OH or −CH3
(f) −CH2OH or −CH=O
(a) −Br
(b) −Br
(c) −CH2CH3
(d) −OH
(e) −CH2OH
(f) −CH=O
(5-7)
Rank each of the following sets of substituents:
(a)
−H, −OH, −CH2CH3, −CH2CH2OH
−OH, −CH2CH2OH, −CH2CH3, −H
(5-7)
Rank each of the following sets of substituents:
(b)
−CO2H, −CO2CH3, −CH2OH, −OH
−OH, −CO2CH3, −CO2H, −CH2OH
(5-7)
Rank each of the following sets of substituents:
(c)
−CN, −CH2NH2, −CH2NHCH3, −NH2
−NH2, −CN, −CH2NHCH3, −CH2NH2
(5-7)
Rank each of the following sets of substituents:
(d)
−SH, −CH2SCH3, −CH3, −SSCH3
−SSCH3, −SH, −CH2SCH3, −CH3
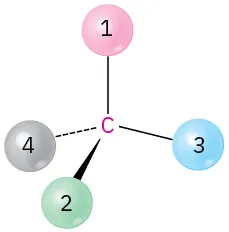
(5-9)
Orient each of the following drawings so that the lowest-ranked group is toward the rear, and then assign R or S configuration:
(a)
S
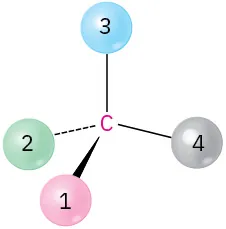
(5-9)
Orient each of the following drawings so that the lowest-ranked group is toward the rear, and then assign R or S configuration:
(b)
R
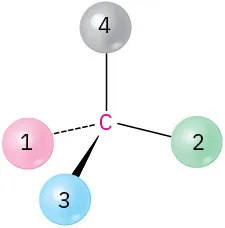
(5-9)
Orient each of the following drawings so that the lowest-ranked group is toward the rear, and then assign R or S configuration:
(c)
S
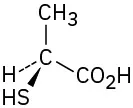
(5-10)
Assign R or S configuration to the chirality center in each of the following molecules:
S
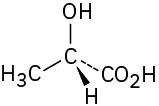
(5-10)
Assign R or S configuration to the chirality center in each of the following molecules:
S
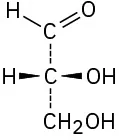
(5-10)
Assign R or S configuration to the chirality center in each of the following molecules:
R
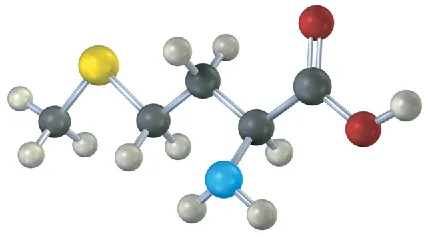
(5-12)
Assign R or S configuration to the chirality center in the following molecular model of the amino acid methionine (blue = N, yellow = S):
S