combustion
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What type of reaction is combustion ? Endo or Exo ?
exothermic reaction as it gives out heat
What are some examples of fossil fuels ?
coal oil and gas which are finite resources
Where can methane be found in ?
natural gas
What is a combustion reaction ?
Combustion is a type of oxidation reaction where water and carbon dioxide is produces and energy is given out
What is the difference between complete and incomplete combustion ?
complete combustion happens with sufficient amount of oxygen and produces carbon dioxide and water
incomplete combustion happens if there is not enough oxygen present, soot (solid carbon) and carbon monoxide are given off (carbon dioxide can also be given off)
What is carbon monoxide ?
a toxic gas which binds preferentially and permanently to the haemoglobin in red blood cells in place of oxygen, this mean d you die of a lack of oxygen
Draw the products of combustion ?
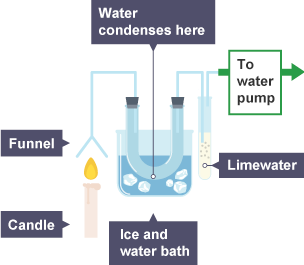
What happens when fuels are burned ? What issues can they cause ?
when burnt, oxygen and nitrogen react together at high temps to produce oxides of nitrogen - these are pollutants and can cause issues linked to acid rain and asthma
How is sulphur dioxide produced ?
sulphur is found in fossil files e.g coal
when burnt its sulphur oxidises into sulphur dioxide SO(lil2)
it then dissolves in the clouds and falls as acid rain
What is the problems with acid rain ?
stunt plant growth
weaken the shells of creatures like shell fish
corrode limestone buildings (see churches/ gravestones)
What are the advantages of using hydrogen, rather than petrol, as fuel in cars ?
it releases more energy per kilogram than any other fuels (except nuclear fuels)
it doesn’t pollute as it only produces water on combustion - no other product is formed
What are the disadvantages of using hydrogen, rather than petrol, as fuel in cars ?
Expensive to produce and requires energy for the production process
Difficult and dangerous to store and move around (usually stored as liquid hydrogen in highly pressurised containers)
The production of hydrogen process releases carbon dioxide
Name non-renewable fossil fuels that are obtained from crude oil and found in natural gas ?
petrol
kerosene
diesel oil
methane - found in natural gas