Tertiary Structure
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
What 2 methods are used to determine the positions of atoms in proteins?
- X-Ray crystallography
- NMR spectroscopy
What determines tertiary structure?
Primary sequence!
What method shows a mobile protein in a solution?
NMR spectroscopy
Are polar or non polar residues typically on the interior of a protein? Exterior?
Interior = non polar
Exterior = polar
Tertiary structure consists of secondary elements that combine to form...
motifs and domains
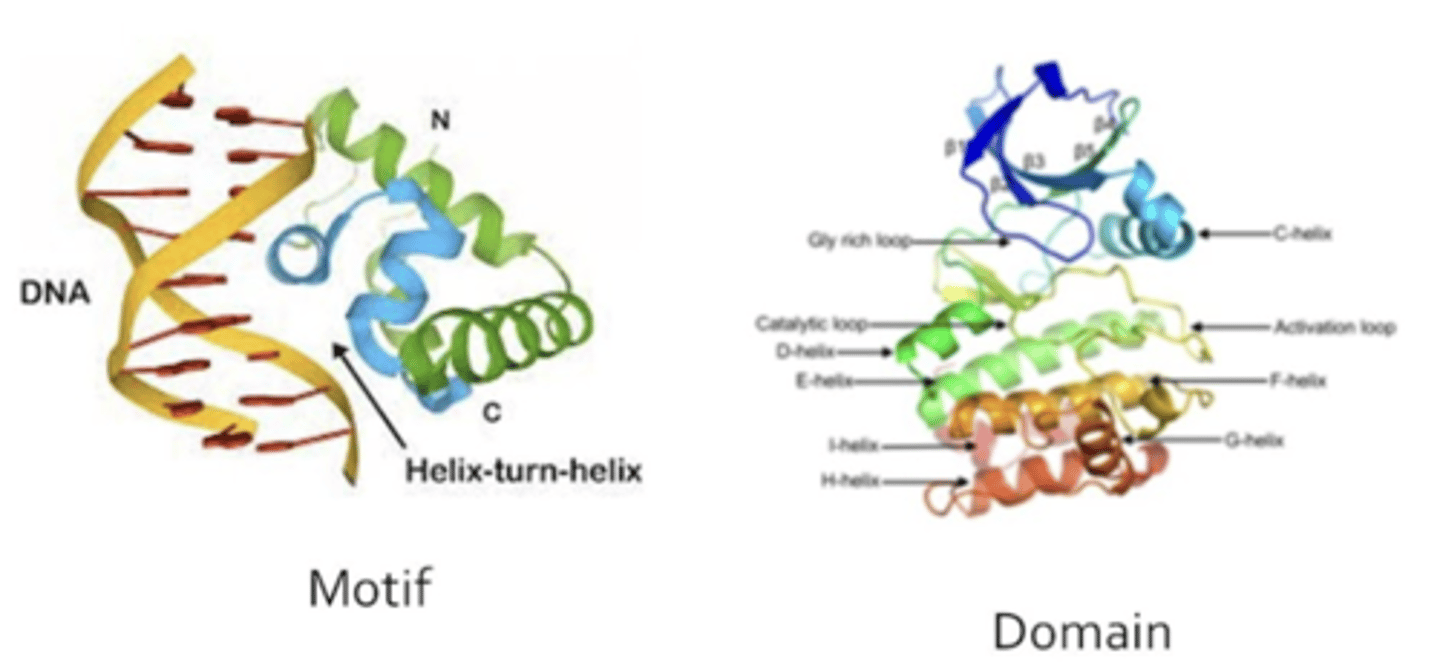
What are motifs? What are domains?
Motif: Small folding topologies found in proteins. Subset of a domain. Cannot fold or function by itself.
Domain: compact, folded protein that are usually stable by themselves in an aqueous solution. Can function alone.
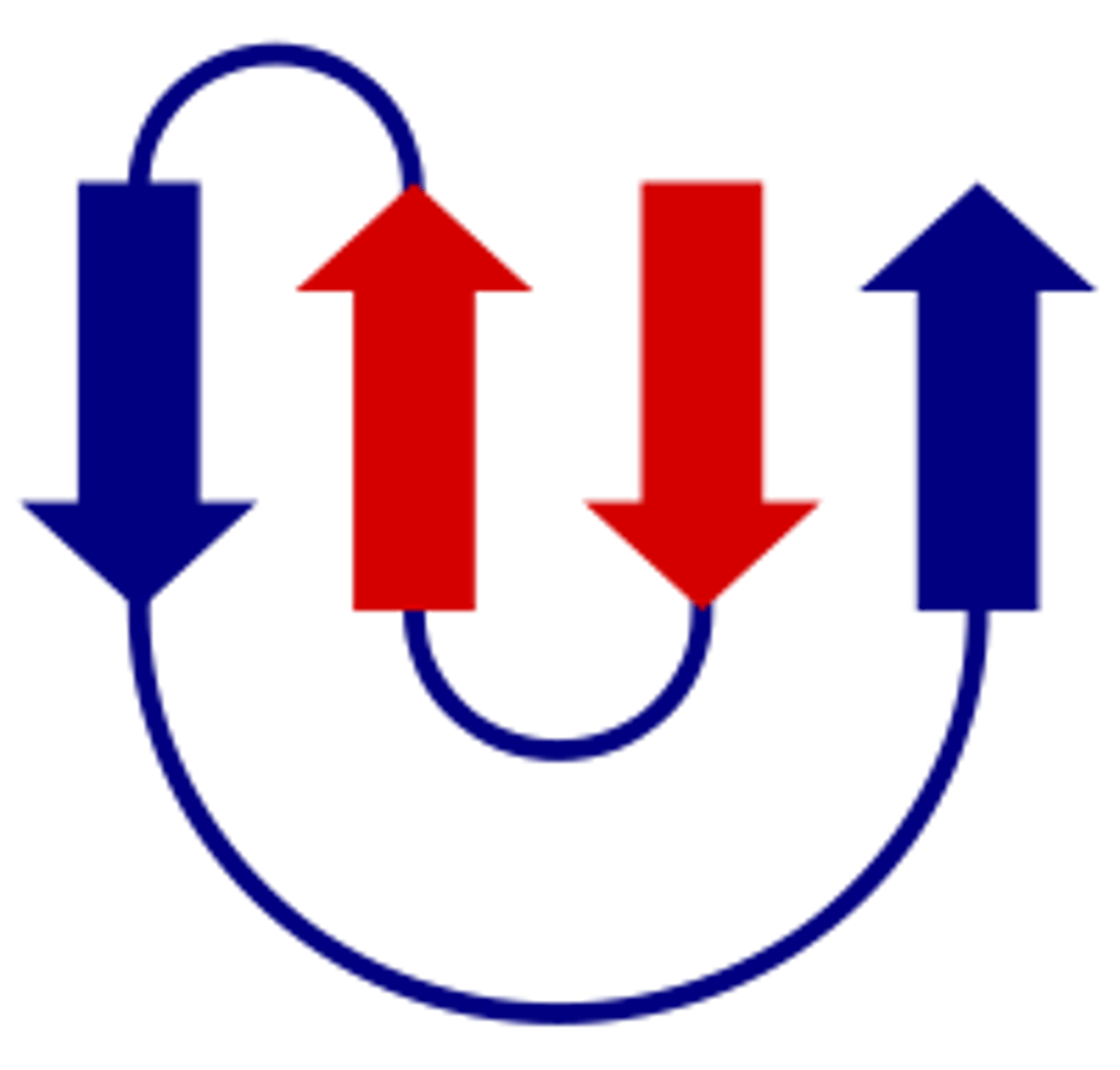
Describe the "greek key" motif
a beta hairpin is folded over to form a 4-stranded antiparallel beta sheet
Is a proteins structure or sequence more highly conserved? What does this mean?
The structure is highly conserved.
There is more than one way to fold a protein!
Where does functional diversity come from in proteins?
- The large number of folded structures that polypeptides can adopt
- Varied chemistry of amino acids/side chains
Describe the structure of a globular protein
- Helices and sheets make up the core
- Polar/hydrophillic faces outside
- most Hydrophobic face interior
- Van der Waals packing is close but not perfect
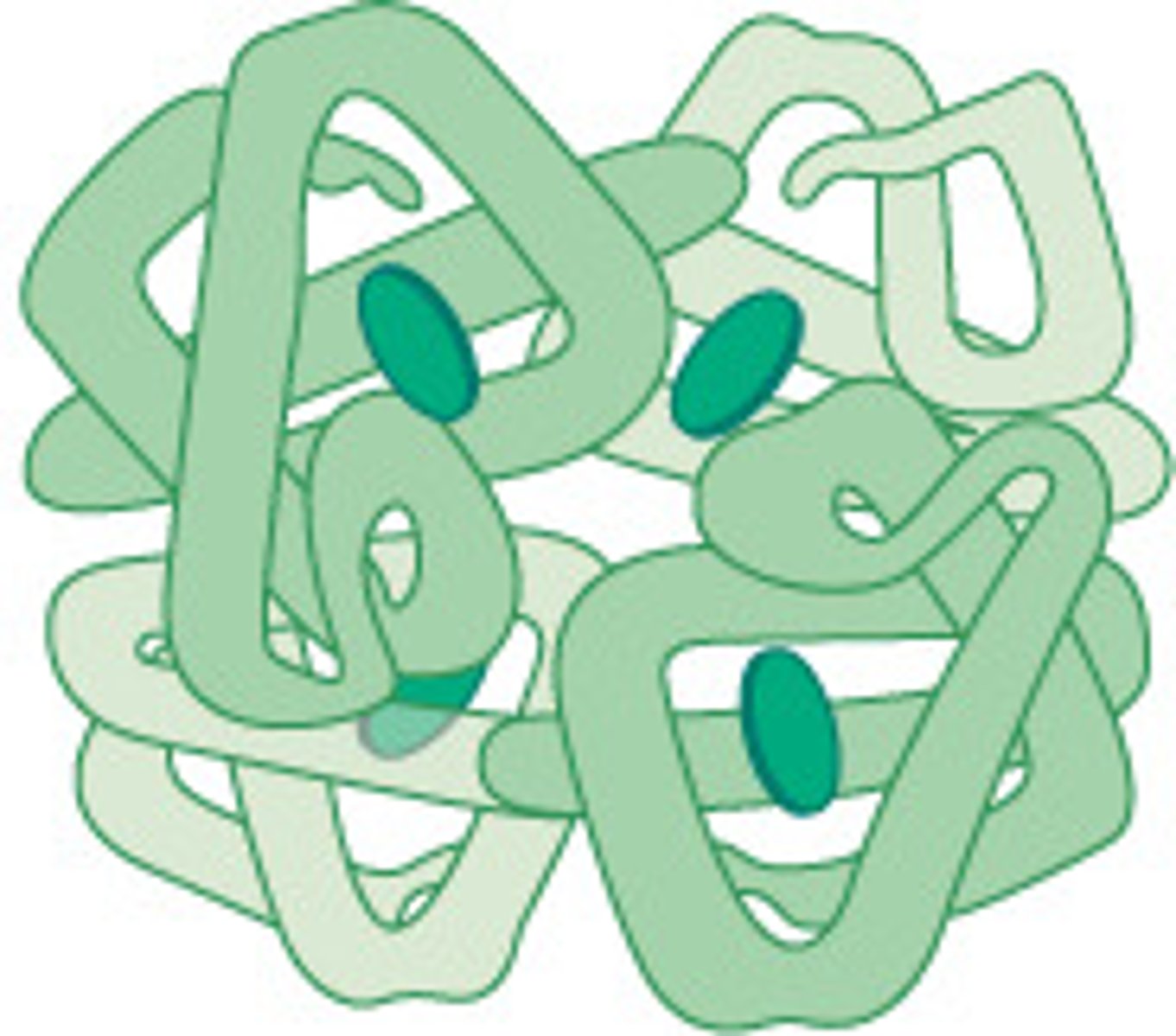
What allows for motion of globular proteins?
Small cavities/empty space
List 4 tertiary structure principles
1. Lots of H-bonds
2. Helices and sheets pack close togeher
3. Peptide segments between secondary structures tend to be short and direct.
4. Proteins fold to form most stable structures.
Where does stability come from in tertiary proteins?
1. Intramolecular hydrogen bonds
2. Compact structure
Why do alpha helices and beta sheets form the core of a globular protein?
- The core is hydrophobic
- N-H and C=O groups must be neutralized in the hydrophobic core
The H-bonded nature of alpha helices and beta sheets is ideal for neutralizing the backbone amides of the hydrophobic core.
Compare/Contrast the protein core vs the protein surface
Core: Helices and sheets are constant and conserved in sequence and structure.
Surface: Short loops, tight turns that connect the helices and sheets of the core. Complex and irregular landscape. Surface interacts with other small molecules needed for functioning.
What is a random coil?
A segment that is not a helix or sheet.
Organized and stable.
Do not conform to any pattern.
Stabilized by side-chain tertiary interactions
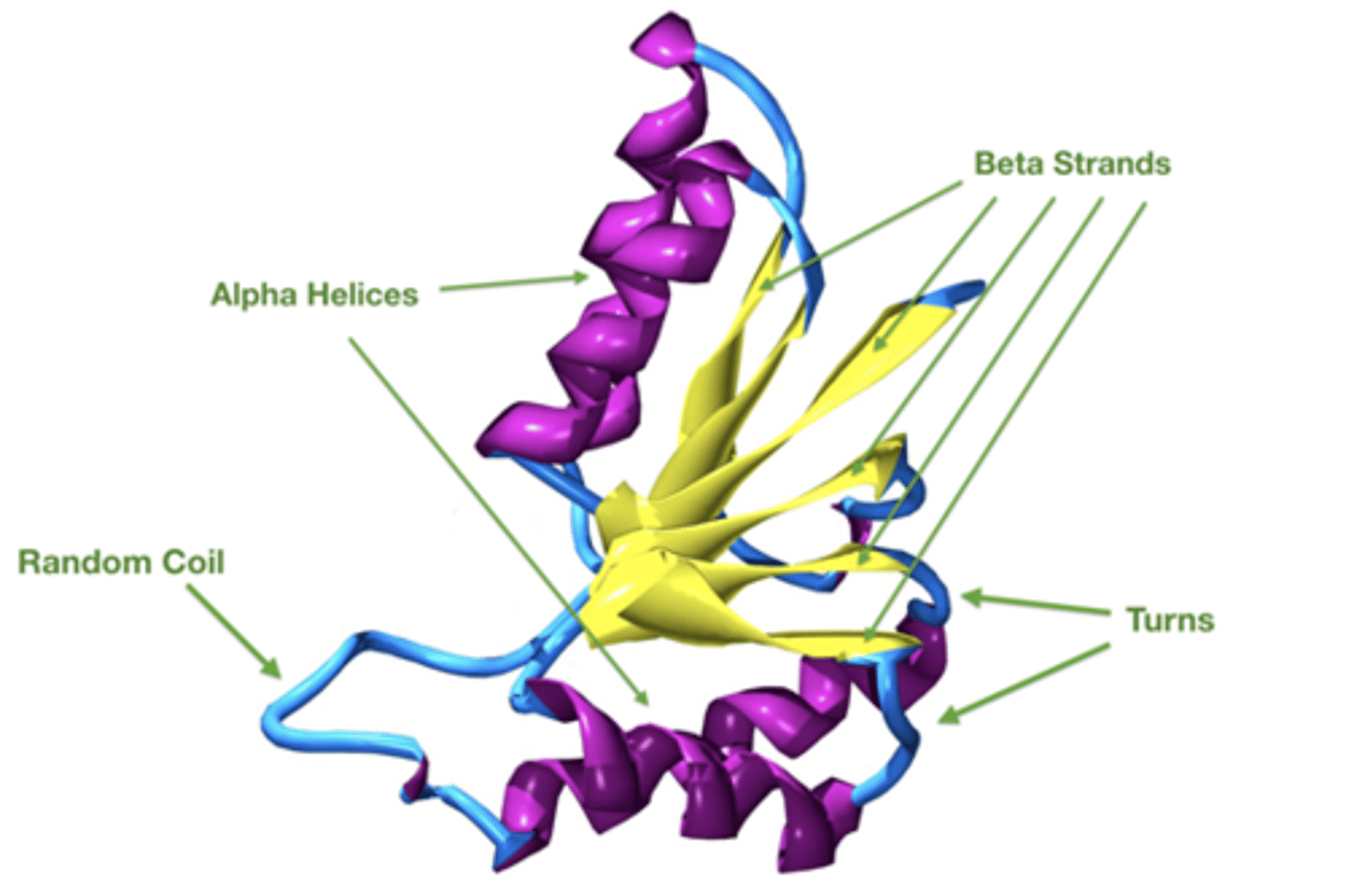
Why is the term "random coil" misleading?
They are actually NOT coiled and NOT random.
Alpha helices on the protein surface are usually _________ while helices on the interior are usually ______.
amphiphilic; hydrophobic
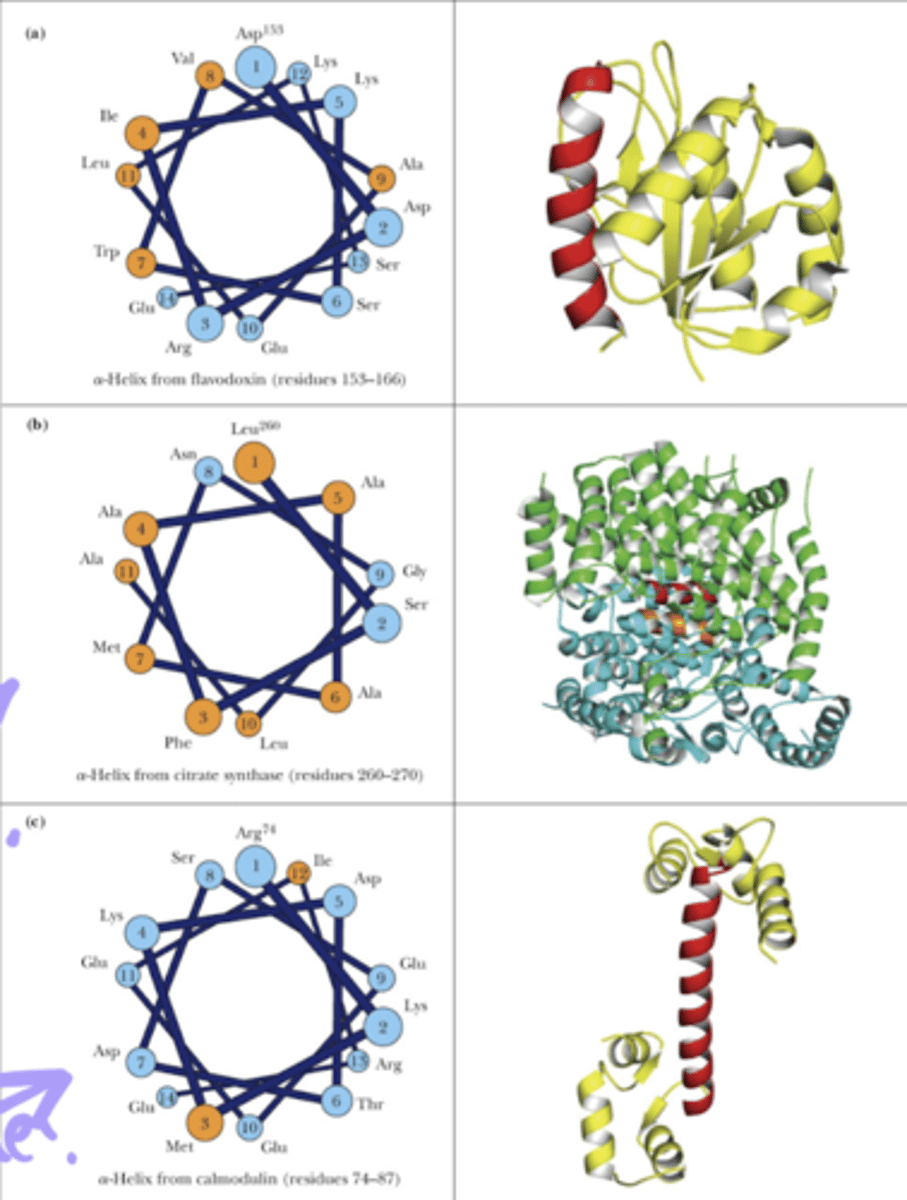
Compare this picture of amphiphilic vs non polar vs polar alpha helices
a. amphiphillic
b. non-polar
c. polar
Multidomains have ________ function(s).
multiple
Why would a protein have multiple copies of the same domain?
To improve that domain's function
What leads to the formation of layers in a protein?
The need to bury hydrophobic residues inside the protein.
Over half of globular proteins have ___ layer(s) of backbone and hydrophobic ____ core(s).
2 layers of backbone; 1 core
What amino acids are most likely to be found in a globular protein?
Those with hydrophobic, non-polar side chains
Ex. Alanine, leucine, valine, isoleucine, and methionine
What are intrinsically disordered proteins?
Proteins that remain unfolded until they dock with an amino acid. (lacks definable structure).
High flexibility. Abundance of polar residues and lack of hydrophobic/nonpolar residues.