Movement of Substances Into & Out of Cells (2d)
1/24
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
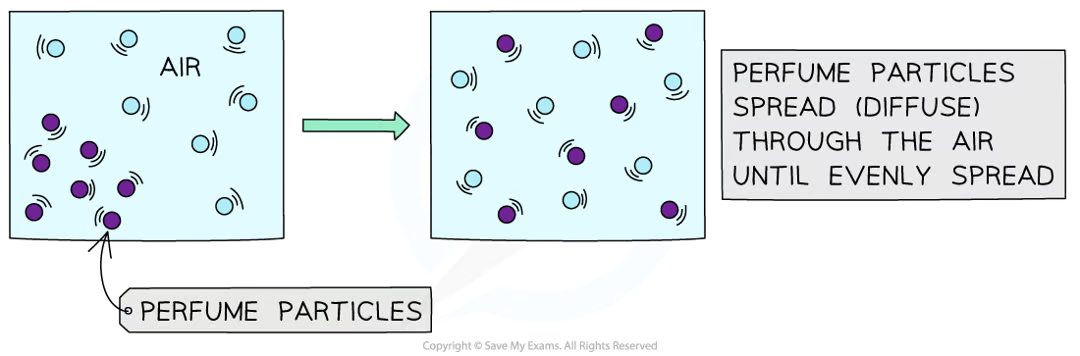
Diffusion
Passive movement of particles from a region of higher to lower concentration, down a concentration gradient
random, but results in spreading out of particles until they are at even conc. throughout the available space
passive since it doesn’t require energy, particles have kinetic energy
Diffusion through the Cell Membrane
gases and small particles move into/out of living cells by diffusion when they cross the cell membrane
partially permeable: allows some gases and molecules but not others
small particles like oxygen can diffuse freely, larger molecules like glucose can’t
Examples of Diffusion in Living Organisms
leaf: conc. of CO2 in chloroplasts is lower than atmosphere when photosynthesising, so CO2 diffuses into the cell
lungs: conc. of oxygen inside the alveoli is higher than conc. of oxygen in the capillaries so oxygen diffuses into the blood to be transported
liver: conc. of urea (waste product) is higher in the liver cells than the blood flowing through the liver, so urea diffuses out of the liver cells into the blood
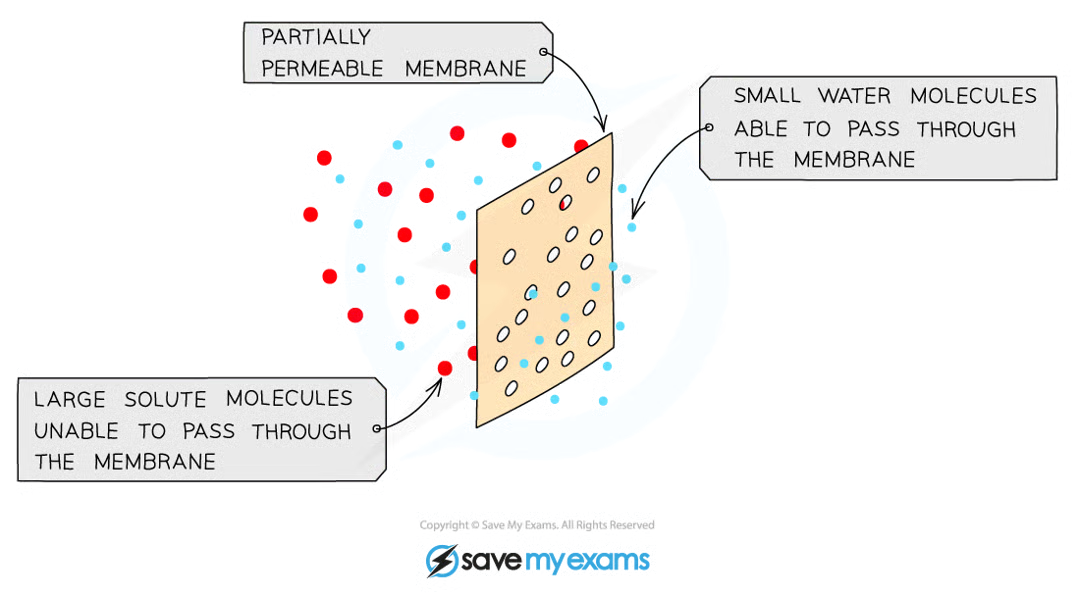
Osmosis
The movement of water from a region of higher water potential (dilute solution) to lower water potential (concentration solution) through a semi-permeable membrane down a concentration gradient
diffusion of water since it’s down the conc. gradient
partially permeable membranes prevent the movement of large molecules (eg: sugars) but allow movement of small water molecules
Water Potential
water moves from high to low water potential
the more solute (eg: sugar or salt) a solution contains, the lower its potential (lower its water concentration)
the lesser the solute, the higher the potential
Pure water has the highest water potential
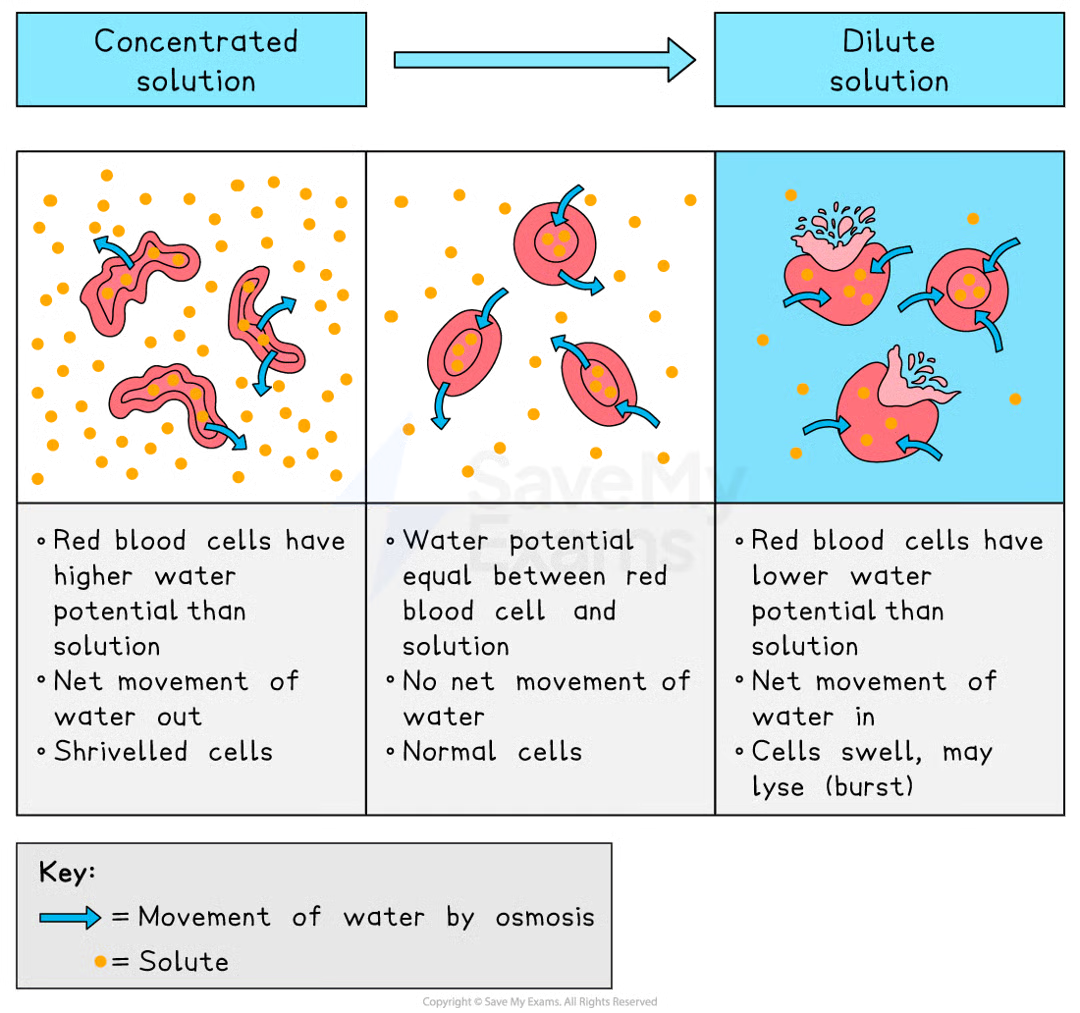
Osmosis in Animal Cells
without a cell wall, osmosis can have sever effects on animal cells
in strong sugar solution (lower water potential) → the cell loses water, becoming crenated (shrivelled)
in distilled water (higher water potential) → the cell gains water, eventually bursting since it lacks a cell wall to maintain structure
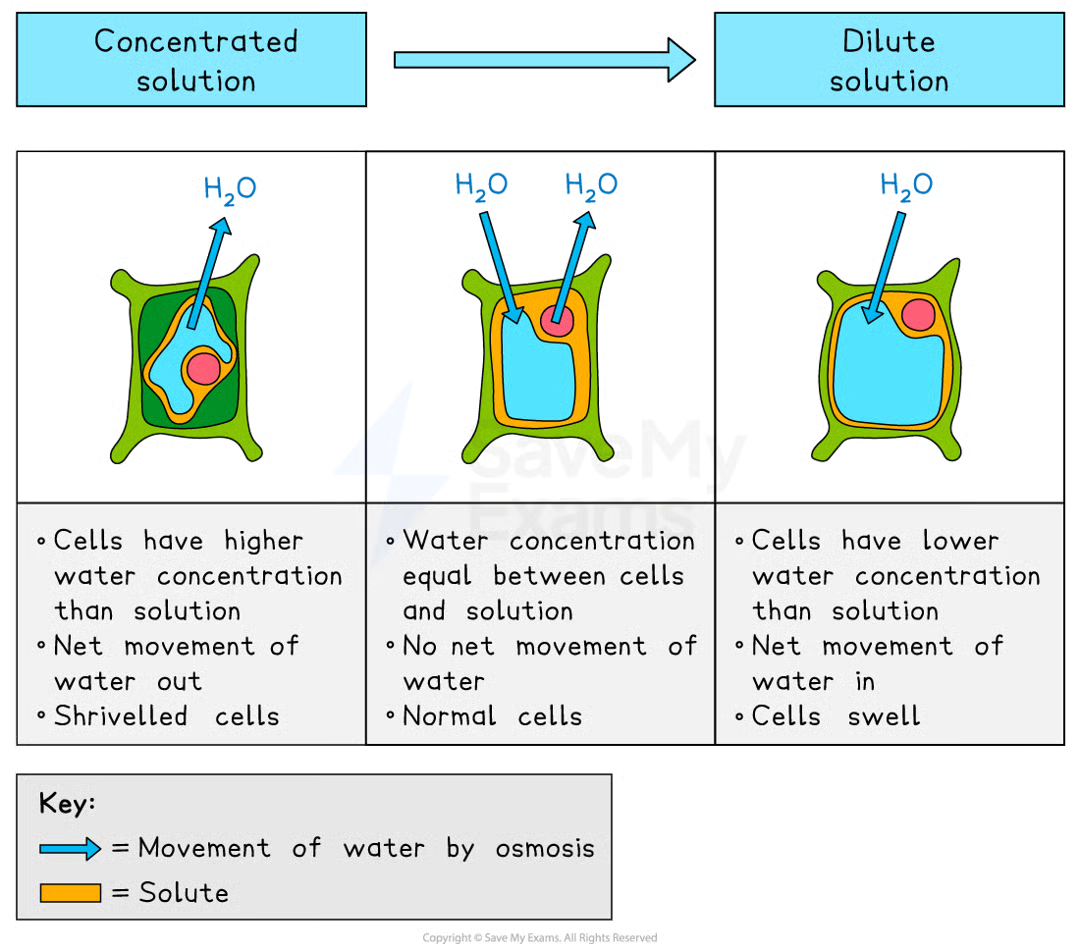
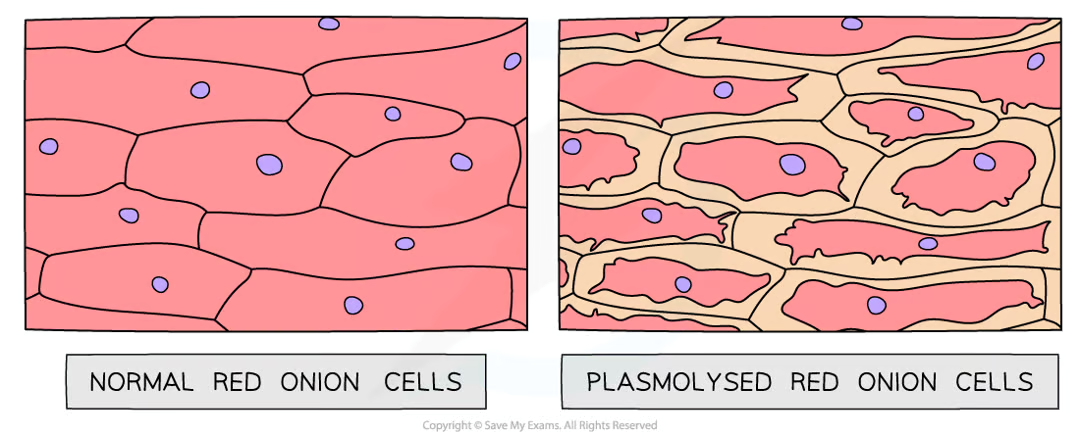
Osmosis in Plant Cells
due to cell wall, plants are protected from bursting
in a strong sugar solution (lower water potential), the cell loses water, the vacuole shrinks and the cell membrane pulls away from the cell wall, making the cell flaccid or plasmolysed
in distilled water (higher water potential), the cell gains water, the vacuole expands, the membrane pushes against the cell wall, making the cell turgid
turgid cells provide structural support and prevent wilting
Active Transport
The movement of particles across a cell membrane from a region of lower to higher concentration, requiring energy released by respiration
energy is needed because particles move against the conc. gradient → released in cellular respiration
involves protein pumps embedded in the cell membrane
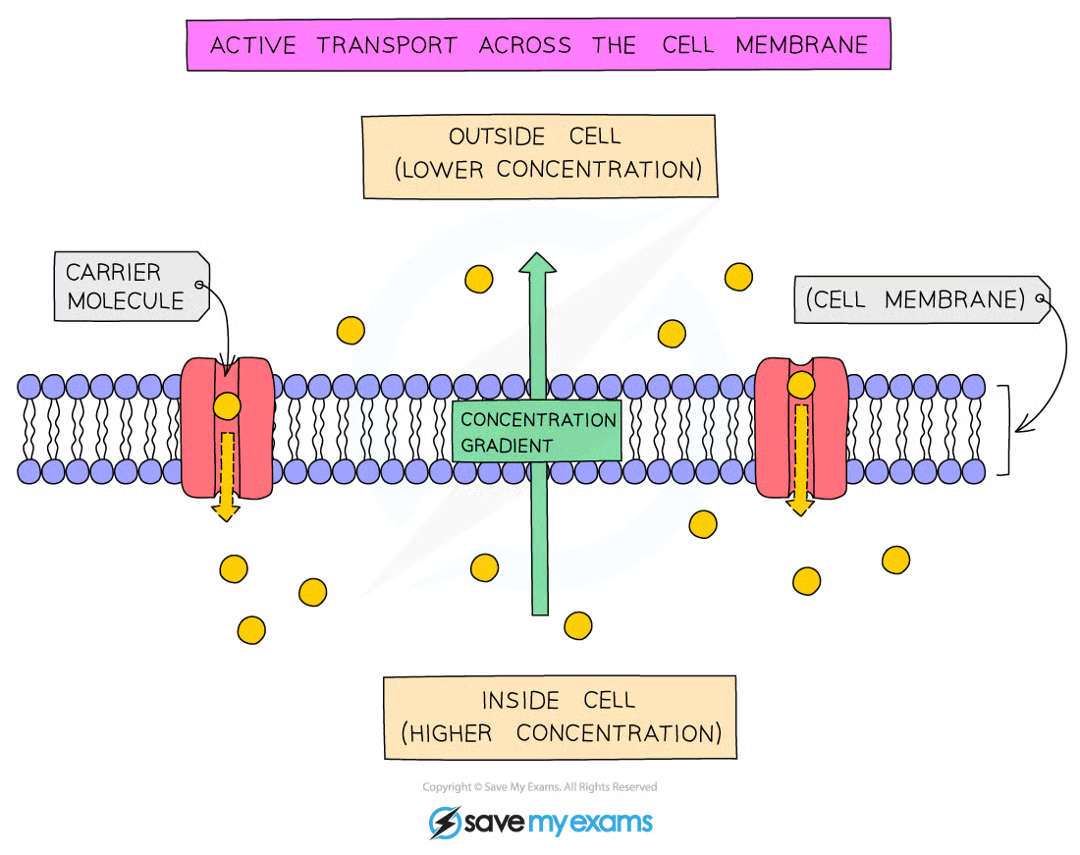
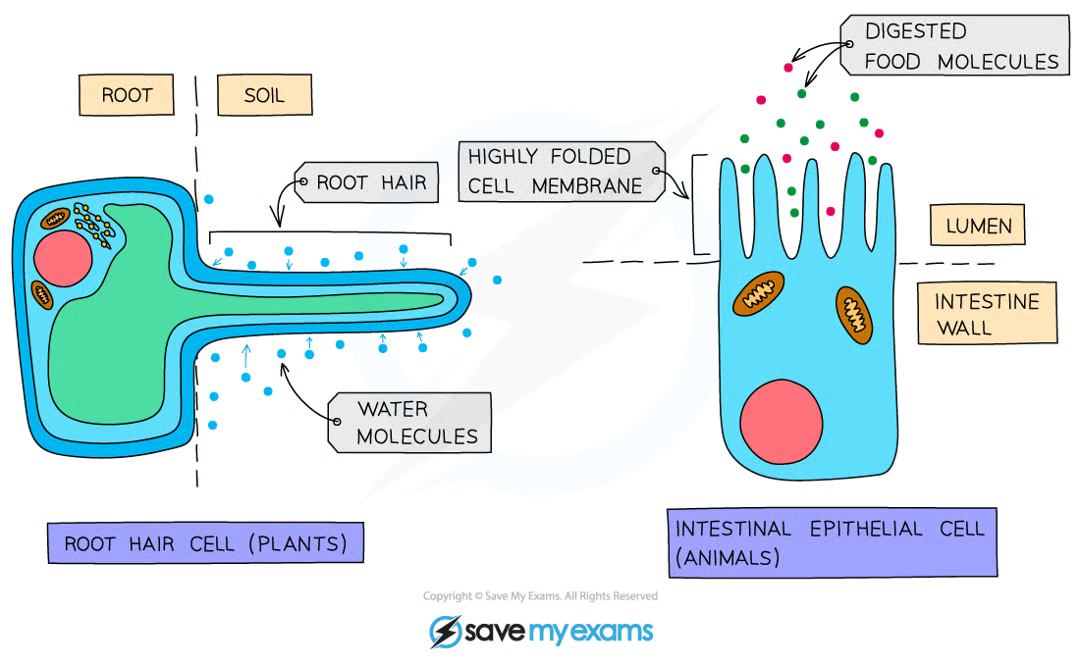
Examples of Active Transport
absorption of products of digestion into the bloodstream form the lumen of the small intestine
absorption of mineral ions from the soil to the root hair cells of plants
Factors influencing Diffusion
surface area to volume ratio
concentration gradient
temperature
distance
Factors influencing Diffusion: Surface Area to Volume Ratio
surface area = total area through which substances can diffuse
as a cell/organism increases in size, its ratio decreases
this means that less surface area is available for each unit of volume, so diffusion alone becomes less efficient at supplying cells with essential substances and removing waste products
to overcome this, larger organisms have evolved specialised exchange surface (alveoli in lungs/ villi in the small intestine) that increase s.a and often have thin walls and good blood supply to maintain steep conc. gradient for efficient diffusion
many organisms adapted for diffusion have increased s.a in some way- eg: root hair cells in plants (which absorb water and mineral ions) and cells lining the ileum in animals (which absorb the products of digestion)
Diffusion Distance
shorter the distance that molecules have to travel, the faster diffusion can occur
this is why alveoli in the lungs and capillary walls are only one cell thick- to minimise diffusion distance for gases
shorter diffusion distance → oxygen and carbon dioxide diffuse rapidly and efficiently between the air in the alveoli and blood in the capillaries
Temperature
higher the temperature, faster the molecules move (have more k.e)
more collisions against the cell membrane, faster rate of movement across them
Concentration gradient
greater the difference in conc. on either side of the membrane, the faster the movement across it will occur
because on the side with the higher concentration, more random collisions against the membrane will occur
Factors influencing Diffusion Practical: Agar Blocks
Agar is clear, jelly-like
when molten agar is mixed with NaOH (an alkali) and phenolphthalein, pink agar is produced
Phenolphthalein is colourless when pH <8.3 and pink in alkaline
pink agar will turn colourless in an acidic solution
as acid diffuses into the blocks, neutralisation occurs between the acid and the alkaline NaOH in the agar block
Factors influencing Diffusion Practical: Apparatus
Pink agar (contains sodium hydroxide and phenolphthalein indicator)
White tile
Scalpel (knife)
Dilute hydrochloric acid
Beakers
Thermometer
Water baths
Ice
Forceps
Stopwatch
Factors influencing Diffusion Practical: Safety Hazards
dilute HCl
scalpel
agar prepared with NaOH and phenolphthalein indicator
Factors influencing Diffusion Practical: Method
using scalpel, cut agar to different side lengths (0.5, 1, 2cm)
calculate SA:V ratio
pour 50ml dilute HCl into a beaker
using forceps, place the 0.5cm cube into the beaker and start a timer
observe, record time when pink agar turns colourless, record time
repeat with same side length to increase reliability
repeat experiment for different side lengths
Factors influencing Diffusion Practical: Results and Analysis
when agar cubes are placed in the HCl, acid diffuses and reacts with the NaOH → pink indicator turns colourless
time taken to turn colourless can be compared
smaller cubes, acid has more sa relative to size → diffusion distance is shorter
as size increases, larger cubes have less sa per unit volume, takes longer for the acid to diffuse to te center → diffusion distance is longer
larger cubes take longer to lose their colour
rate of diffusion remains constant, but total rate of exchange depends on cube’s SA:V ratio
experiment models how small organisms with high SA:V ratio rely on diffusion alone for gas exchange, larger organisms cannot
Factors influencing Diffusion Practical: Limitations
determining endpoint introduces human error
improvement: measure distance in mm after a set period of time → provides qualitative measurement that’s easier to compare
difficult to cut cubes into the same size and small differences affect SA”V ratio and rate of diffusion
improvement: use a ruler and sharp scalpel to cut as accurately as possible
Factors influencing Osmosis Practical: Apparatus
Potatoes
Cork borer
Knife
Sucrose solutions (from 0 Mol/dm3 to 1 mol/dm3)
Test tubes
Balance
Paper towels
Ruler
Test tube rack
Factors influencing Osmosis Practical: Method
prepare a range of sucrose solutions ranging from 0 Mol/dm3 (distilled water) to 1 mol/dm3
set up 6 labelled test tubes with 10cc of each sucrose solution
using the knife, cork borer and ruler, cut 6 equally sized cylinders of potato
blot with paper towel and weigh on the balance
put 1 piece into each conc. of sucrose solution
after 4 hours, remove, blot and reweigh
Factors influencing Osmosis Practical: Results and Analysis
potato in distilled water gains most mass because of a high conc. gradient → water moves in by osmosis, increasing turgor pressure, making the potato firm
potato in strongest solution loses the most mass as water moves out by osmosis → making cells flaccid and the potato turns soft
if there was no net movement, conc. was same as in the cytoplasm of the potato cells
Factors influencing Osmosis Practical: Limitations
slight differences in cylinders make the results unreliable
solution: for each sucrose conc., repeat with several cylinders. repeating = anomalies can be identified and ignored when calculating mean
Factors influencing Osmosis Practical: CORMMS
C - changing the concentration of sucrose solution
O - the potato cylinders will all be taken from the same potato or potatoes of the same age
R - repeat the investigation several times to ensure results are reliable
M1 - measure the change in mass of the potato cylinders
M2 - ...after 4 hours
S - control the volume of sucrose solution used, the dimensions of the potato cylinders and each cylinder must be blotted before it is weighed each time