tests for cations and anions
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
How to test for GROUP 2 IONS
From Mg to Ba
Add NaOH (increase in solubility as you go down)
Add H2SO4 (decrease in solubility as you go down)
How to test for ammonium ions
add NaOH
NH4+ + OH- → NH3 (g) + H2O
Turns red litmus paper blue
How to test for GROUP 7 IONS (1)
Option 1: Acidified silver nitrate
Chloride ions- white precipitate
Bromide ions- cream precipitate
Iodide ions- yellow precipitate
Then to further identify, add dilute NH3:
Chlorine will dissolve
Bromine will stay the same
Then add concentrated NH3:
Bromine will dissolve
iodine will remain same for both
How to test for group 7 ions (2)
All acid base reactions first. Then bromide and iodide react redox after
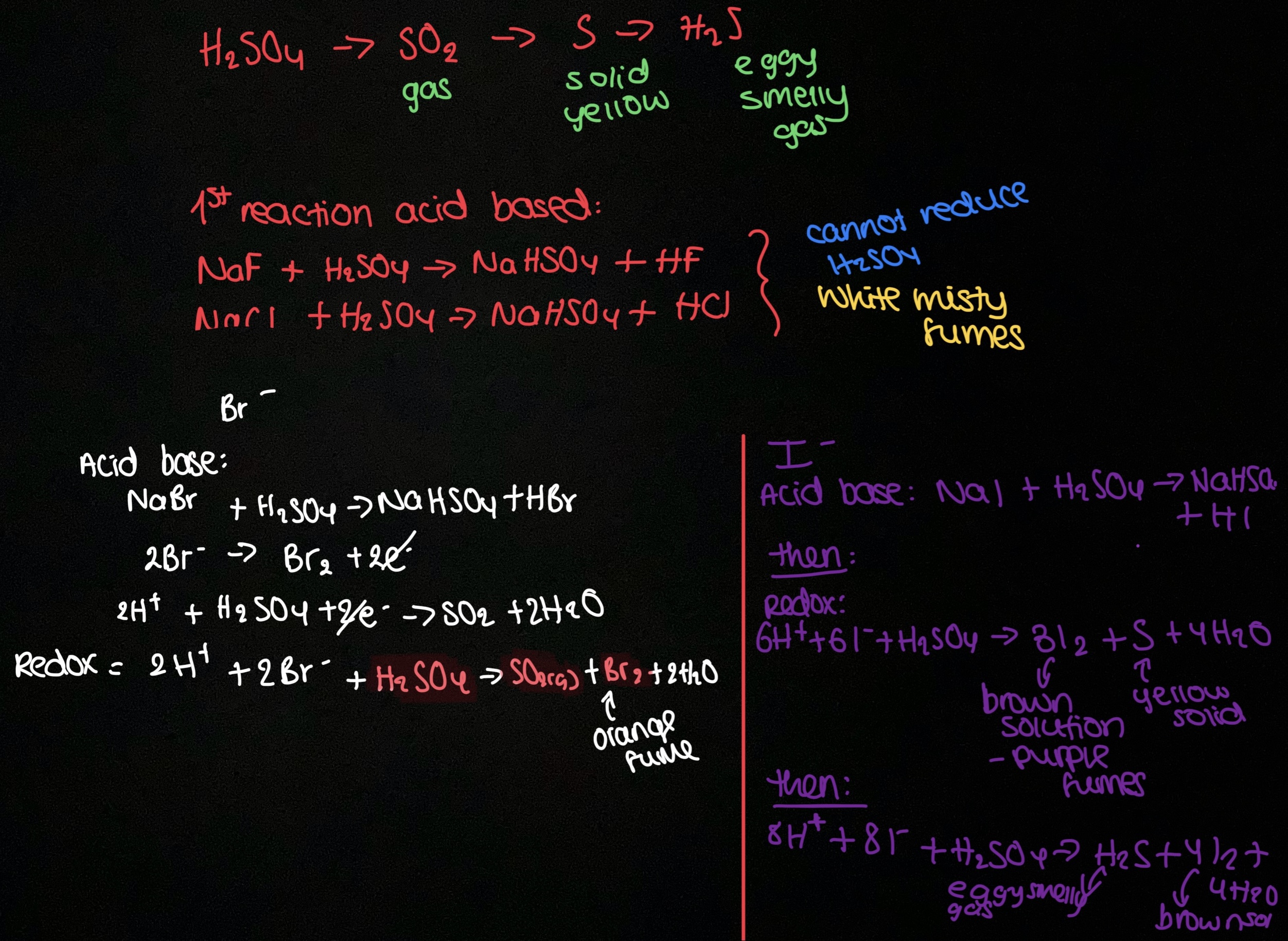
How to test for carbonate ions
add acid
Effervescence (bubbled through limewater which can make it cloudy)
How to test for sulphate ions
add acidified barium chloride
Ba 2+ + SO4 2- → BaSO4 (s) which is a white precipitate
How to test for hydroxide ions
Turns damp red litmus paper blue