Chemistry IGCSE Edexcel Triple science specification as flashcards
0.0(0)
0.0(0)
Card Sorting
1/307
Earn XP
Description and Tags
TRIPLE : Triple content only EXTRAS: extra Information that I think is useful to know
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
308 Terms
1
New cards
1) Principles of Chemistry
(a) States of matter
(b) Elements, compounds and mixtures
(c) Atomic structure
(d) The Periodic Table
(e) Chemical formulae, equations and calculations
(f) Ionic bonding
(g) Covalent bonding
(h) Metallic bonding
(i) Electrolysis
(b) Elements, compounds and mixtures
(c) Atomic structure
(d) The Periodic Table
(e) Chemical formulae, equations and calculations
(f) Ionic bonding
(g) Covalent bonding
(h) Metallic bonding
(i) Electrolysis
2
New cards
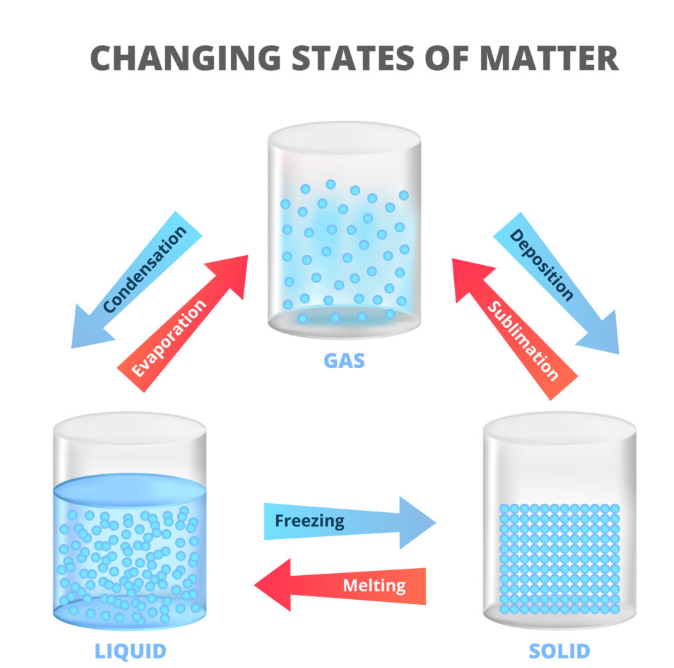
What are the three states of matter
Solid
Liquids
Gases
Liquids
Gases
3
New cards
the states of matter in terms of the arrangement, movement and energy of the particles
Solids
Solids
regular arrangement
vibrate about a fixed position
the particles are very close
vibrate about a fixed position
the particles are very close
4
New cards
the states of matter in terms of the arrangement, movement and energy of the particles
Liquid
Liquid
Randomly arranged
move around each other
close together
move around each other
close together
5
New cards
the states of matter in terms of the arrangement, movement and energy of the particles
gas
gas
randomly arranged
move quickly in all directions
far apart
move quickly in all directions
far apart
6
New cards
the names of all the interconversions
Melting
Boiling
Freezing
Evaporation
Condensation
Sublimation
Boiling
Freezing
Evaporation
Condensation
Sublimation
7
New cards
How is melting achieved
* The process requires heat energy which transforms into **kinetic** energy, allowing the particles to move
* It occurs at a specific temperature known as the **melting** **point** which is **unique** to each pure solid
\
* It occurs at a specific temperature known as the **melting** **point** which is **unique** to each pure solid
\
8
New cards
How is Boiling achieved
* Boiling is when a liquid changes into a gas
* This requires heat which causes bubbles of gas to form **below** the surface of a liquid, allowing for liquid particles to escape from the surface and from within the liquid
* It occurs at a specific temperature known as the **boiling** **point** which is **unique** to each pure liquid
* This requires heat which causes bubbles of gas to form **below** the surface of a liquid, allowing for liquid particles to escape from the surface and from within the liquid
* It occurs at a specific temperature known as the **boiling** **point** which is **unique** to each pure liquid
9
New cards
How is freezing achieved
* Freezing is when a liquid changes into a solid
* This is the reverse of melting and occurs at exactly the **same** **temperature** as melting, hence the melting point and freezing point of a pure substance are the same
* Water for example freezes and melts at 0 ºC
\
* It requires a significant decrease in temperature (or loss of thermal energy) and occurs at a specific temperature which is **unique** for each pure substance
\
* This is the reverse of melting and occurs at exactly the **same** **temperature** as melting, hence the melting point and freezing point of a pure substance are the same
* Water for example freezes and melts at 0 ºC
\
* It requires a significant decrease in temperature (or loss of thermal energy) and occurs at a specific temperature which is **unique** for each pure substance
\
10
New cards
How is evaporation achieved
* When a liquid changes into a gas
* Evaporation occurs only at the **surface** of liquids where high energy particles can escape from the liquids surface at **low** temperatures, below the boiling point of the liquid
* The larger the surface area and the warmer the liquid/surface, the more quickly a liquid can evaporate
* Evaporation occurs over a **range** of temperatures, but heating will speed up the process as particles need energy to escape from the surface
\
* Evaporation occurs only at the **surface** of liquids where high energy particles can escape from the liquids surface at **low** temperatures, below the boiling point of the liquid
* The larger the surface area and the warmer the liquid/surface, the more quickly a liquid can evaporate
* Evaporation occurs over a **range** of temperatures, but heating will speed up the process as particles need energy to escape from the surface
\
11
New cards
how is condensation achieved
* When a gas changes into a liquid, usually on cooling
* When a gas is cooled its particles lose energy and when they bump into each other, they lack energy to bounce away again, instead grouping together to form a liquid
* When a gas is cooled its particles lose energy and when they bump into each other, they lack energy to bounce away again, instead grouping together to form a liquid
12
New cards
How is sublimation achieved
* When a solid changes directly into a gas
* This happens to only a few solids, such as iodine or solid carbon dioxide
* The reverse reaction also happens and is called desublimation or deposition
\
\
\
* This happens to only a few solids, such as iodine or solid carbon dioxide
* The reverse reaction also happens and is called desublimation or deposition
\
\
\
13
New cards
How the results of experiments involving the dilution of coloured solutions can be explained
**Description:**
* When potassium magnate (VII) crystals are dissolved in water, the solution can be diluted several times
* The colour fades but does not disappear until a lot of dilutions have been done
**Explanation:**
* This indicates that there are a lot of particles in a small amount of potassium manganate (VII) and therefore the particles must be very small
\
\
\
* When potassium magnate (VII) crystals are dissolved in water, the solution can be diluted several times
* The colour fades but does not disappear until a lot of dilutions have been done
**Explanation:**
* This indicates that there are a lot of particles in a small amount of potassium manganate (VII) and therefore the particles must be very small
\
\
\
14
New cards
how are the results of experiments involving the diffusion of gases can be explained
**Description:**
* Here, we see the diffusion of bromine gas from one gas jar to another
* After 5 minutes the bromine gas has diffused from the bottom jar to the top jar
**Explanation:**
* The air and bromine particles are moving randomly and there are large gaps between particles
* The particles can therefore easily mix together
* Here, we see the diffusion of bromine gas from one gas jar to another
* After 5 minutes the bromine gas has diffused from the bottom jar to the top jar
**Explanation:**
* The air and bromine particles are moving randomly and there are large gaps between particles
* The particles can therefore easily mix together
15
New cards
know what is meant by the term: • solvent
The liquid in which a solute dissolves
e.g. The water in sea water
e.g. The water in sea water
16
New cards
know what is meant by the term: • solute
The substance which dissolves in a liquid to form a solution
e.g. the salt in seawater
e.g. the salt in seawater
17
New cards
know what is meant by the term: • solution
The mixture formed when a solute is dissolved in a solvent
e.g. seawater
e.g. seawater
18
New cards
know what is meant by the term: •saturated solution
A solution with the maximum concentration of solute dissolved into the solvent
e.g. Sea water in the dead sea
e.g. Sea water in the dead sea
19
New cards
EXTRA’S: know what is meant by the term: • soluble
Describes a substance that will dissolve
e.g. Salt is soluble in water
e.g. Salt is soluble in water
20
New cards
EXTRA’S: know what is meant by the term: • insoluble
Describes a substance that won’t dissolve
e.g. sand is soluble in water
e.g. sand is soluble in water
21
New cards
TRIPLE:
what is meant by the term solubility in the units g per 100 g of solvent
what is meant by the term solubility in the units g per 100 g of solvent
Solubility is **a measurement of how much of a substance will dissolve in a given volume of a liquid**
* Different substances have different solubilities
* Solubility can be expressed in **g per 100 g of solvent**
* Solubility of solids is affected by temperature
* As **temperature increases**, solids usually become **more soluble**
* Different substances have different solubilities
* Solubility can be expressed in **g per 100 g of solvent**
* Solubility of solids is affected by temperature
* As **temperature increases**, solids usually become **more soluble**
22
New cards
TRIPLE: how to plot and interpret solubility curves
* Solubility graphs or curves represent solubility in g per 100 g of water plotted against temperature
* To plot a solubility curve, the maximum mass of solvent that can be dissolved in 100 g of water before a saturated solution is formed, is determined at a series of different temperatures
\
***Solubility curve for three salts. While the solubility of most salts increases with temperature, sodium chloride, or common salt, hardly changes at all***
\
\
* To plot a solubility curve, the maximum mass of solvent that can be dissolved in 100 g of water before a saturated solution is formed, is determined at a series of different temperatures
\
***Solubility curve for three salts. While the solubility of most salts increases with temperature, sodium chloride, or common salt, hardly changes at all***
\
\
23
New cards
TRIPLE/PRACTICAL: investigate the solubility of a solid in water at a specific
temperature
temperature
1. Prepare a two beakers, one as a hot water bath and one as an ice bath
2. Using a small measuring cylinder, measure out 4 cm3 of distilled water into a boiling tube.
3. On a balance weigh out 2.6 g of ammonium chloride and add it to the boiling tube
4. Place the boiling tube into the hot water bath and stir until the solid dissolves
5. Transfer the boiling tube to the ice bath and allow it to cool while stirring
6. Note the temperature at which crystals first appear and record it in a table of results
7. Add 1 cm3 of distilled water then warm the solution again to dissolve the crystals
8. Repeat the cooling process again noting the temperature at which crystals first appear.
9. Continue the steps until a total of 10 cm3 of water has been added
24
New cards
understand how to classify a substance as an element,
a substance made up of atoms that all contain the same number of protons (one type of atom) and cannot be split into anything simpler. There are 118 known elements.
e.g. hydrogen, oxygen, carbon
e.g. hydrogen, oxygen, carbon
25
New cards
understand how to classify a substance as a compound
A pure substance made up of two or more elements chemically combined together. There are unlimited types of compounds. Cannot be separated to physical methods of separation.
e.g. copper (ll) sulphate, calcium carbonate
e.g. copper (ll) sulphate, calcium carbonate
26
New cards
understand how to classify a substance as a mixture
A combination of two or more substances (elements and/or compounds) that are not chemically joined together. Can be separated by physical methods of separation.
e.g. salt and water, air
e.g. salt and water, air
27
New cards
How to distinguish a pure substance
* Pure substances melt and boil at **specific** and **sharp** temperatures e.g. pure water has a boiling point of 100 °C and a melting point of 0 °C
* Mixtures have a **range** of melting and boiling points as they consist of **different** substances that tend to lower the melting point and broaden the melting point range
\
* The closer the measured value is to the actual melting or boiling point then the purer the sample is
* Mixtures have a **range** of melting and boiling points as they consist of **different** substances that tend to lower the melting point and broaden the melting point range
\
* The closer the measured value is to the actual melting or boiling point then the purer the sample is
28
New cards
What is simple distillation ?
* This is used to separate a liquid and **soluble solid** from a solution (e.g., water from a solution of salt water) or a pure liquid from a mixture of liquids
\
* The solution is heated, and pure water evaporates producing a vapour which rises through the neck of the round bottomed flask
* The vapour passes through the condenser, where it cools and condenses, turning into the pure liquid that is collected in a beaker
* After all the water is evaporated from the solution, only the solid solute will be left behind
\
* The solution is heated, and pure water evaporates producing a vapour which rises through the neck of the round bottomed flask
* The vapour passes through the condenser, where it cools and condenses, turning into the pure liquid that is collected in a beaker
* After all the water is evaporated from the solution, only the solid solute will be left behind
29
New cards
What is fractional distillation ?
* This is used to separate two or more liquids that are **miscible** with one another (e.g., ethanol and water from a mixture of the two)
* The solution is heated to the temperature of the substance with the lowest boiling point
* This substance will rise and evaporate first, and vapours will pass through a condenser, where they cool and condense, turning into a liquid that will be collected in a beaker
* All of the substance is evaporated and collected, leaving behind the other components(s) of the mixture
* For water and ethanol
* Ethanol has a boiling point of 78 ºC and water of 100 ºC
* The mixture is heated until it reaches 78 ºC, at which point the ethanol boils and distills out of the mixture and condenses into the beaker
\
* When the temperature starts to increase to 100 ºC heating should be stopped. Water and ethanol are now separated
* The solution is heated to the temperature of the substance with the lowest boiling point
* This substance will rise and evaporate first, and vapours will pass through a condenser, where they cool and condense, turning into a liquid that will be collected in a beaker
* All of the substance is evaporated and collected, leaving behind the other components(s) of the mixture
* For water and ethanol
* Ethanol has a boiling point of 78 ºC and water of 100 ºC
* The mixture is heated until it reaches 78 ºC, at which point the ethanol boils and distills out of the mixture and condenses into the beaker
\
* When the temperature starts to increase to 100 ºC heating should be stopped. Water and ethanol are now separated
30
New cards
What is filtration
* Used to separate an **undissolved solid** from a mixture of the solid and a liquid / solution ( e.g., sand from a mixture of sand and water)
* Centrifugation can also be used for this mixture
\
* A piece of filter paper is placed in a filter funnel above a beaker
* A mixture of insoluble solid and liquid is poured into the filter funnel
* The filter paper will only allow small liquid particles to pass through as filtrate
* Solid particles are too large to pass through the filter paper so will stay behind as a residue
\
\
* Centrifugation can also be used for this mixture
\
* A piece of filter paper is placed in a filter funnel above a beaker
* A mixture of insoluble solid and liquid is poured into the filter funnel
* The filter paper will only allow small liquid particles to pass through as filtrate
* Solid particles are too large to pass through the filter paper so will stay behind as a residue
\
\
31
New cards
What is crystallisation ?
* Used to separate a **dissolved solid** from a solution, when the solid is much more soluble in hot solvent than in cold (e.g., copper sulphate from a solution of copper (II) sulphate in water)
* The solution is heated, allowing the solvent to evaporate, leaving a saturated solution behind
* Test if the solution is saturated by dipping a clean, dry, cold glass rod into the solution
* If the solution is saturated, crystals will form on the glass rod
\
* The saturated solution is allowed to cool slowly
* Crystals begin to grow as solids will come out of solution due to decreasing solubility
* The crystals are collected by filtering the solution, they are washed with cold distilled water to remove impurities and are then allowed to dry
\
\
* The solution is heated, allowing the solvent to evaporate, leaving a saturated solution behind
* Test if the solution is saturated by dipping a clean, dry, cold glass rod into the solution
* If the solution is saturated, crystals will form on the glass rod
\
* The saturated solution is allowed to cool slowly
* Crystals begin to grow as solids will come out of solution due to decreasing solubility
* The crystals are collected by filtering the solution, they are washed with cold distilled water to remove impurities and are then allowed to dry
\
\
32
New cards
What is paper chromatography
* This technique is used to separate substances that have **different** **solubilities** in a given solvent (e.g., different coloured inks that have been mixed to make black ink)
* A **pencil** **line** is drawn on chromatography paper and spots of the sample are placed on it. Pencil is used for this as ink would run into the chromatogram along with the samples
* The paper is then lowered into the solvent container, making sure that the pencil line sits **above** the level of the solvent, so the samples don’t wash into the solvent container
* The paper is called the **stationary phase**
* The solvent travels up the paper by **capillary action**, taking some of the coloured substances with it; it is called the **mobile phase**
* Different substances have different solubilities so will travel at different rates, causing the substances to spread apart
* Those substances with higher solubility will travel further than the others
\
* This will show the different components of the ink / dye
* If two or more substances are the same, they will produce identical chromatograms
* If the substance is a mixture, it will separate on the paper to show all the different components as separate spots
* An impure substance will show up with more than one spot, a pure substance should only show up with one spot
* A **pencil** **line** is drawn on chromatography paper and spots of the sample are placed on it. Pencil is used for this as ink would run into the chromatogram along with the samples
* The paper is then lowered into the solvent container, making sure that the pencil line sits **above** the level of the solvent, so the samples don’t wash into the solvent container
* The paper is called the **stationary phase**
* The solvent travels up the paper by **capillary action**, taking some of the coloured substances with it; it is called the **mobile phase**
* Different substances have different solubilities so will travel at different rates, causing the substances to spread apart
* Those substances with higher solubility will travel further than the others
\
* This will show the different components of the ink / dye
* If two or more substances are the same, they will produce identical chromatograms
* If the substance is a mixture, it will separate on the paper to show all the different components as separate spots
* An impure substance will show up with more than one spot, a pure substance should only show up with one spot
33
New cards
how does a chromatogram provides information about the composition of a mixture
* Pure substances will produce only **one spot** on the chromatogram
* If two or more substances are the same, they will produce identical chromatograms
* If the substance is a **mixture**, it will separate on the paper to show all the **different** **components** as **separate** spots
* An impure substance therefore will produce a chromatogram with more than one spot
* If two or more substances are the same, they will produce identical chromatograms
* If the substance is a **mixture**, it will separate on the paper to show all the **different** **components** as **separate** spots
* An impure substance therefore will produce a chromatogram with more than one spot
34
New cards
how do you use the calculation of Rf values to identify the components of a mixture
* These values are used to **identify** the components of mixtures
* The *R*f value of a particular compound is always the **same** but it is dependent, however, on the solvent used
* If the solvent is changed then the value changes
* Calculating the *R*f value allows chemists to **identify unknown substances** because it can be compared with *R*f values of known substances under the same conditions
* These values are known as **reference values**
**Calculation**
* The Retention factor is found using the following calculation:
\
**Rf = distance travelled by substance ÷ distance travelled by solvent**
\
* The Rf value will always lie between 0 and 1; the closer it is to 1, the more soluble is that component in the solvent
* The *R*f value is a ratio and therefore has no units
* The *R*f value of a particular compound is always the **same** but it is dependent, however, on the solvent used
* If the solvent is changed then the value changes
* Calculating the *R*f value allows chemists to **identify unknown substances** because it can be compared with *R*f values of known substances under the same conditions
* These values are known as **reference values**
**Calculation**
* The Retention factor is found using the following calculation:
\
**Rf = distance travelled by substance ÷ distance travelled by solvent**
\
* The Rf value will always lie between 0 and 1; the closer it is to 1, the more soluble is that component in the solvent
* The *R*f value is a ratio and therefore has no units
35
New cards
investigate paper chromatography using inks/food colourings
1. Use a ruler to draw a horizontal pencil line 2 cm from the end of the chromatography paper
2. Use a different capillary tube to put a tiny spot of each colouring A, B, C and D on the line
3. Use the fifth tube to put a small spot of the unknown mixture U on the line
4. Make sure each spot is no more than 2-3 mm in diameter and label each spot in pencil
5. Pour water into the beaker to a depth of no more than 1 cm and clip the top of the chromatography paper to the wooden spill. The top end is the furthest from the spots
6. Carefully rest the wooden spill on the top edge of the beaker. The bottom edge of the paper
should dip into the solvent
7. Allow the solvent to travel undisturbed at least three quarters of the way up the paper
8. Remove the paper and draw another pencil line on the dry part of the paper as close to the wet edge as possible. This is called the solvent front line
9. Measure the distance in mm between the two pencil lines. This is the distance travelled by the water solvent
10. For each of food colour A, B, C and D measure the distance in mm from the start line to the middle of the spot
36
New cards
What is meant by the term atom
the smallest particles of an element that consists of electrons surrounding a nucleus that contains protons and nuetrons
37
New cards
What is meant by the term molecule
A group of tow or more atoms chemically joined together forming of identifiable unit which retains the properties and composition of the substance
38
New cards
What is the structure of an proton in terms of
\-position
\-relative mass
\-relative charges of subatomic particles
\-position
\-relative mass
\-relative charges of subatomic particles
inside with the neutron
1
\+1
1
\+1
39
New cards
What is the structure of an neutron in terms of
\-position
\-relative mass
\-relative charges of subatomic particles
\-position
\-relative mass
\-relative charges of subatomic particles
inside with the proton
1
0 (neutral)
1
0 (neutral)
40
New cards
What is the structure of an electron in terms of
\-position
\-relative mass
\-relative charges of subatomic particles
\-position
\-relative mass
\-relative charges of subatomic particles
on the outside
1/1840
\-1
1/1840
\-1
41
New cards
What does atomic number mean
The number of protons in the nucleus of an atom
42
New cards
What does mass number mean
The sum of the number of protons and nuetrons in the nucleus of an atom
43
New cards
What does isotopes mean
Atoms of the same element contain the same number of protons and electrons but a different number of neutrons. Therefore, they have the same atomic number but a different mass number
44
New cards
What does Relative atomic mass mean
Weighted average mass of one atom of an element, taking into account the abundance of all the isotopes of that element. It is measured as a ration 1/12 of the mass of an atom of carbon -12
45
New cards
How do you calculate the relative atomic mass of an element (Ar) from isotopic abundances
Ar = (% of isotope A X Mass of isotope A) + (%of isotope B X mass of isotope B) / 100
\
if there is more than two isotopes just extend the equation on the top line
\
if there is more than two isotopes just extend the equation on the top line
46
New cards
How are elements arranged in the periodic table in order of atomic number
* Elements are arranged on the periodic table in order of **increasing atomic number**
* Each element has one proton **more** than the element preceding it
* This is done so that elements end up in columns with other elements which have similar properties
* Each element has one proton **more** than the element preceding it
* This is done so that elements end up in columns with other elements which have similar properties
47
New cards
How are elements arranged in the periodic table in order of periods
* The table is arranged in vertical columns called **groups** and in rows called **periods**
* **Period:** These are the horizontal rows that show the number of shells of electrons an atom has and are numbered from 1 - 7
* E.g. elements in period 2 have two electron shells, elements in period 3 have three electron shells
* **Period:** These are the horizontal rows that show the number of shells of electrons an atom has and are numbered from 1 - 7
* E.g. elements in period 2 have two electron shells, elements in period 3 have three electron shells
48
New cards
How are elements arranged in the periodic table in order of groupds
* **Group:** These are the vertical columns that show how many outer electrons each atom has and are numbered from 1 – 7, with a final group called group 0 (instead of group 8)
* E.g. group 4 elements have atoms with 4 electrons in the outermost shell, group 6 elements have atoms with 6 electrons in the outermost shell and so on
* E.g. group 4 elements have atoms with 4 electrons in the outermost shell, group 6 elements have atoms with 6 electrons in the outermost shell and so on
49
New cards
EXTRA : what is an electronic configuration
The electron configuration of an element describes how electrons are distributed in its atomic orbitals.
\
* Electrons orbit the nucleus in shells and each shell has a different amount of energy associated with it
* The further away from the nucleus, the more energy a shell has
\
* Electrons orbit the nucleus in shells and each shell has a different amount of energy associated with it
* The further away from the nucleus, the more energy a shell has
50
New cards
how do you deduce the electronic configurations of the first 20 elements from their positions in the Periodic Table
first shell holds 2
second and on holds 8
outer shell can hold any from 1-8
\
to work it out look at the atomic number
take 2 , take 8 , take 8 etc
if you can’t take 8 the number that is left is the number on the outer shell
\
(this makes sense to me i’m not sure how else to explain it)
second and on holds 8
outer shell can hold any from 1-8
\
to work it out look at the atomic number
take 2 , take 8 , take 8 etc
if you can’t take 8 the number that is left is the number on the outer shell
\
(this makes sense to me i’m not sure how else to explain it)
51
New cards
how do you use electrical conductivity and the acid-base character of oxides to classify elements as metals or non-metals
Metals are good conductors of electricity
Non metals are poor conductors of electricity
\
Many metals react with acids
Non metals do not react with acids
Non metals are poor conductors of electricity
\
Many metals react with acids
Non metals do not react with acids
52
New cards
EXTRA: how do you know if something is a metal based on its electron arrangement
metals have 1-3 outer shell electrons
Non metals have 4-7 in the outer shell
Non metals have 4-7 in the outer shell
53
New cards
How do you identify an element as a metal or a non-metal according to its position in the Periodic Table
Metals are on the left the last metals are Al , Ge , Sb , Po
The Non metals solids start after the metals and finish with bromine and sulphur
although nitrogen is on the row nitrogen is a gas
the rest are non metal gases
The Non metals solids start after the metals and finish with bromine and sulphur
although nitrogen is on the row nitrogen is a gas
the rest are non metal gases
54
New cards
how do the electronic configuration of a main group element is related to its position in the Periodic Table
* Elements in the same group in the periodic table will have similar chemical properties
* This is because they have the same number of outer electrons so will react and bond similarly
\
* We can use the group number to predict how elements will react as the number of valence shell electrons in an element **influences** how the element reacts.
* Therefore, elements in the same group react **similarly**
* By observing the reaction of one element from a group, you can predict how the other elements in that group will react
\
* The group 1 metals become **more reactive** as you move down the group while the group 7 metals show a **decrease** in reactivity moving down the group
* This is because they have the same number of outer electrons so will react and bond similarly
\
* We can use the group number to predict how elements will react as the number of valence shell electrons in an element **influences** how the element reacts.
* Therefore, elements in the same group react **similarly**
* By observing the reaction of one element from a group, you can predict how the other elements in that group will react
\
* The group 1 metals become **more reactive** as you move down the group while the group 7 metals show a **decrease** in reactivity moving down the group
55
New cards
why do elements in the same group of the Periodic Table have similar chemical properties
* This is because they have the same number of outer electrons so will react and bond similarly
56
New cards
why do the noble gases (Group 0) do not readily react
* They are all non-metal, **monatomic** (exist as single atoms), **colourless**, **non-flammable** gases at room temperature
* The group 0 elements all have **full outer shells** of electrons; this electronic configuration is **extremely** **stable**
* Elements participate in reactions to complete their outer shells by losing, gaining, or sharing **electrons**
* The Group 0 elements do not need to do this, because of their full outer shells which makes them unreactive and **inert**
\
* Other than helium which has 2 electrons in its outer shell, the noble gases have eight valence electrons (which is why you may see this group labelled “group 8”
* The group 0 elements all have **full outer shells** of electrons; this electronic configuration is **extremely** **stable**
* Elements participate in reactions to complete their outer shells by losing, gaining, or sharing **electrons**
* The Group 0 elements do not need to do this, because of their full outer shells which makes them unreactive and **inert**
\
* Other than helium which has 2 electrons in its outer shell, the noble gases have eight valence electrons (which is why you may see this group labelled “group 8”
57
New cards
write word equations :
• for reactions studied in this specification
• for unfamiliar reactions where suitable information is provided.
• for reactions studied in this specification
• for unfamiliar reactions where suitable information is provided.
* The reactants are those substances on the **left-hand side** of the arrow and can be thought of as the chemical **ingredients** of the reaction
* They react with each other and form new substances
* The products are the new substances which are on the **right-hand side** of the arrow
* The arrow (which is spoken as “*goes to*” or “*produces*”) implies the **conversion** of reactants into products
* Reaction **conditions** or the name of a **catalyst** (a substance added to make a reaction go faster) can be written above the arrow
* An example is the reaction of sodium hydroxide (a base) and hydrochloric acid produces sodium chloride (common table salt) and water:
\
**sodium hydroxide + hydrochloric acid ⟶ sodium chloride + water**
\
\
* They react with each other and form new substances
* The products are the new substances which are on the **right-hand side** of the arrow
* The arrow (which is spoken as “*goes to*” or “*produces*”) implies the **conversion** of reactants into products
* Reaction **conditions** or the name of a **catalyst** (a substance added to make a reaction go faster) can be written above the arrow
* An example is the reaction of sodium hydroxide (a base) and hydrochloric acid produces sodium chloride (common table salt) and water:
\
**sodium hydroxide + hydrochloric acid ⟶ sodium chloride + water**
\
\
58
New cards
* Chemical equations use the chemical symbols of each reactant and product
* When balancing equations, there has to be the **same number of atoms** of each element on either side of the equation in accordance with the Law of Conservation of Mass
* A symbol equation uses the formulae of the reactants and products to show what happens in a chemical reaction
* A symbol equation must be balanced to give the correct ratio of reactants and products:
\
**S + O2 → SO2**
\
* This equation shows that one atom of sulfur (S) reacts with one molecule of oxygen (O2) to make one molecule of sulfur dioxide (SO2)
* The following non-metals must be written as molecules: H2, N2, O2, F2, Cl2, Br2 and I2
* To balance an equation you work across the equation from left to right, checking one element after another
* If there is a group of atoms, for example a nitrate group (NO3–) that has not changed from one side to the other, then count the whole group as one entity rather than counting the individual atoms
* Examples of chemical equations:
* Acid-base neutralisation reaction:
\
\
**NaOH (aq) + HCl (aq) ⟶ NaCl (aq) + H2O (l)**
* Redox reaction:
\
\
**2Fe2O3 (aq) + 3C (s) ⟶ 4Fe (s) + 3CO2 (g)**
\
* In each equation there are equal numbers of each atom on either side of the reaction arrow so the equations are balanced
\
\
\
* Don't forget to add state symbols when writing balanced equations:
* (s) solid
* (l) liquid
* (g) gas
* (aq) aqueous
* When balancing equations, there has to be the **same number of atoms** of each element on either side of the equation in accordance with the Law of Conservation of Mass
* A symbol equation uses the formulae of the reactants and products to show what happens in a chemical reaction
* A symbol equation must be balanced to give the correct ratio of reactants and products:
\
**S + O2 → SO2**
\
* This equation shows that one atom of sulfur (S) reacts with one molecule of oxygen (O2) to make one molecule of sulfur dioxide (SO2)
* The following non-metals must be written as molecules: H2, N2, O2, F2, Cl2, Br2 and I2
* To balance an equation you work across the equation from left to right, checking one element after another
* If there is a group of atoms, for example a nitrate group (NO3–) that has not changed from one side to the other, then count the whole group as one entity rather than counting the individual atoms
* Examples of chemical equations:
* Acid-base neutralisation reaction:
\
\
**NaOH (aq) + HCl (aq) ⟶ NaCl (aq) + H2O (l)**
* Redox reaction:
\
\
**2Fe2O3 (aq) + 3C (s) ⟶ 4Fe (s) + 3CO2 (g)**
\
* In each equation there are equal numbers of each atom on either side of the reaction arrow so the equations are balanced
\
\
\
* Don't forget to add state symbols when writing balanced equations:
* (s) solid
* (l) liquid
* (g) gas
* (aq) aqueous
59
New cards
EXTRA : how to balance equations
The best approach is to practice lot of examples of balancing equations
* By trial and error change the coefficients (multipliers) in front of the formulae, one by one checking the result on the other side
* Balance elements that appear on their own, last in the process
* By trial and error change the coefficients (multipliers) in front of the formulae, one by one checking the result on the other side
* Balance elements that appear on their own, last in the process
60
New cards
calculate relative formula masses (including relative molecular masses) (Mr) from relative atomic masses (Ar)
* We have seen previously that the symbol for the relative atomic mass is *Ar*
* To calculate the *Mr* of a substance, you have to add up the **relative atomic masses** of all the atoms present in the formula
\
n = little number after Chemical symbol symbol
\
(n x Chemical symbol Ar ) + (n x chemical symbol Ar) = Mr
* To calculate the *Mr* of a substance, you have to add up the **relative atomic masses** of all the atoms present in the formula
\
n = little number after Chemical symbol symbol
\
(n x Chemical symbol Ar ) + (n x chemical symbol Ar) = Mr
61
New cards
What is a mole
* One mole of a substance contains the same number of the stated particles, atoms, molecules, or ions as one mole of any other substance
\
in short hand the unit for the amount of a substance
\
in short hand the unit for the amount of a substance
62
New cards
moles equation
moles = mass/mr
\
\
\
\
63
New cards
calculate reacting masses using experimental data and chemical equations
* Chemical equations can be used to calculate the **moles** or **masses** of reactants and products
* To do this, information given in the question is used to find the amount in moles of the substances being considered
* Then, the **ratio** between the substances is identified using the balanced chemical equation
* Once the moles have been determined they can then be converted into grams using the relative atomic or relative formula masses
* To do this, information given in the question is used to find the amount in moles of the substances being considered
* Then, the **ratio** between the substances is identified using the balanced chemical equation
* Once the moles have been determined they can then be converted into grams using the relative atomic or relative formula masses
64
New cards
How so you calculate percentage yield
percentage yield = (actual yield/theoretical yield) x 100
\
* The **actual yield** is the recorded amount of product obtained
* The **theoretical yield** is the amount of product that would be obtained under perfect practical and chemical conditions
\
\
* The **actual yield** is the recorded amount of product obtained
* The **theoretical yield** is the amount of product that would be obtained under perfect practical and chemical conditions
\
65
New cards
understand how the formulae of simple compounds can be obtained experimentally, water and salts containing water of crystallisation
**Aim:**
* To determine the formula of hydrated copper sulfate, CuSO4. *x*H2O
\
* Measure the mass of evaporating dish
* Add a known mass of hydrated salt
* Heat over a Bunsen burner, gently stirring, until the blue salt turns completely white, indicating that all the water has been lost
* Record the mass of the evaporating dish and its contents
\
***Mass of the white anhydrous salt:***
* Measure the mass of white anhydrous salt remaining
***Mass of water:***
* Subtract the mass of the white anhydrous salt remaining from the mass of known hydrated salt
* **Step 1 –** Divide the mass of the copper sulfate and the water by their respective molar masses
* **Step 2 –** Simplify the ratio of water to copper sulfate:
* **Step 3 –** Represent the ratio in the form ‘salt.xH2O’
* To determine the formula of hydrated copper sulfate, CuSO4. *x*H2O
\
* Measure the mass of evaporating dish
* Add a known mass of hydrated salt
* Heat over a Bunsen burner, gently stirring, until the blue salt turns completely white, indicating that all the water has been lost
* Record the mass of the evaporating dish and its contents
\
***Mass of the white anhydrous salt:***
* Measure the mass of white anhydrous salt remaining
***Mass of water:***
* Subtract the mass of the white anhydrous salt remaining from the mass of known hydrated salt
* **Step 1 –** Divide the mass of the copper sulfate and the water by their respective molar masses
* **Step 2 –** Simplify the ratio of water to copper sulfate:
* **Step 3 –** Represent the ratio in the form ‘salt.xH2O’
66
New cards
what is meant by the term empirical formula
* The **empirical** **formula** is the simplest whole number ratio of the atoms of each element present in one molecule or formula unit of the compound
* E.g. the empirical formula of ethanoic acid is CH2O
* E.g. the empirical formula of ethanoic acid is CH2O
67
New cards
What is meant by the terms molecular formula
* The **molecular** **formula** is the formula that shows the **number** and **type** of each atom in a molecule
* E.g. the molecular formula of ethanoic acid is C2H4O2
* E.g. the molecular formula of ethanoic acid is C2H4O2
68
New cards
How do you calculate empirical formula
For example, the molecular formula of glucose is C 6H 12O 6 but the empirical formula is CH 2O.
69
New cards
How do you calculate molecular formula
* Divide the relative formula mass of the molecular formula by the relative formula mass of the empirical formula
* Multiply the number of each element present in the empirical formula by this number to find the molecular formula
* Multiply the number of each element present in the empirical formula by this number to find the molecular formula
70
New cards
worked example of how to do molecular formula
\
The empirical formula of **X** is C4H10S1 and the relative formula mass of X is 180. What is the molecular formula of **X**? \n **Relative atomic masses:** carbon : 12 hydrogen : 1 sulfur : 32
\
The empirical formula of **X** is C4H10S1 and the relative formula mass of X is 180. What is the molecular formula of **X**? \n **Relative atomic masses:** carbon : 12 hydrogen : 1 sulfur : 32
**Step 1 -** Calculate the relative formula mass of the empirical formula
\
(C x 4) + (H x 10) + (S x 1) = (12 x 4) + (1 x 10) + (32 x 1) = 90
\
**Step 2 -** Divide the relative formula mass of X by the mass of the empirical formula
\
180 / 90 = 2
\
**Step 3 -** Multiply each number of elements by 2
\
(C4 x 2) + (H10 x 2) + (S1 x 2) = (C8) + (H20) + (S2)
\
**Molecular Formula of X =** C8H20S2
\
(C x 4) + (H x 10) + (S x 1) = (12 x 4) + (1 x 10) + (32 x 1) = 90
\
**Step 2 -** Divide the relative formula mass of X by the mass of the empirical formula
\
180 / 90 = 2
\
**Step 3 -** Multiply each number of elements by 2
\
(C4 x 2) + (H10 x 2) + (S1 x 2) = (C8) + (H20) + (S2)
\
**Molecular Formula of X =** C8H20S2
71
New cards
TRIPLE: how do you carry out calculations involving amount of substance, volume and concentration (in mol/dm3) of solution
concentration = moles/volume in dm3
\
if its in cm3
moles = conc x vol/1000
conc = moles x 1000/vol
\
To go from cm3 to dm3 : divide by 1000
\
To go from dm3 to cm3 : multiply by 1000
\
if its in cm3
moles = conc x vol/1000
conc = moles x 1000/vol
\
To go from cm3 to dm3 : divide by 1000
\
To go from dm3 to cm3 : multiply by 1000
72
New cards
TRIPLE: how do you carry out calculations involving gas volumes and the molar volume of a gas
* At room temperature and pressure, the volume occupied by one mole of any gas was found to be **24** dm3 or **24,000** cm3
* This is known as the **molar gas volume at RTP**
* RTP stands for “room temperature and pressure” and the conditions are **20 ºC** and **1 atmosphere** (atm)
\
amount of gas (moles) = volume (dm3)/24 (dm3 mol -1)
\
amount of gas (moles) = volume (cm3)/24 ,000 (cm3 mol -1)
* This is known as the **molar gas volume at RTP**
* RTP stands for “room temperature and pressure” and the conditions are **20 ºC** and **1 atmosphere** (atm)
\
amount of gas (moles) = volume (dm3)/24 (dm3 mol -1)
\
amount of gas (moles) = volume (cm3)/24 ,000 (cm3 mol -1)
73
New cards
PRACTICAL: know how to determine the formula of a metal oxide by combustion (e.g. magnesium oxide) or by reduction (e.g. copper(II) oxide)
to determine the empirical formula of magnesium oxide by combustion of magnesium
\
**Method:**
* Measure mass of crucible with lid
* Add sample of magnesium into crucible and measure mass with lid (calculate the mass of the metal by subtracting the mass of empty crucible)
* Strongly heat the crucible over a Bunsen burner for several minutes
* Lift the lid frequently to allow sufficient air into the crucible for the magnesium to fully oxidise without letting magnesium oxide smoke escape
* Continue heating until the mass of crucible remains constant (maximum mass), indicating that the reaction is complete
* Measure the mass of crucible and contents (calculate the mass of metal oxide by subtracting the mass of empty crucible)
\
**Working out the empirical formula:**
***Mass of metal:***
Subtract mass of crucible from magnesium and the mass of the empty crucible***Mass of oxygen:***
Subtract mass of the magnesium used from the mass of magnesium oxide
**Step 1 –** Divide each of the two masses by the relative atomic masses of the elements
**Step 2 –** Simplify the ratio
**magnesium** **oxygen**
Mass a b
Mole a / Ar b / Ar
= x = y
Ratio x : y
**Step 3 –** Represent the ratio into the form ‘MxOy‘ E.g, MgO
\
\
**Method:**
* Measure mass of crucible with lid
* Add sample of magnesium into crucible and measure mass with lid (calculate the mass of the metal by subtracting the mass of empty crucible)
* Strongly heat the crucible over a Bunsen burner for several minutes
* Lift the lid frequently to allow sufficient air into the crucible for the magnesium to fully oxidise without letting magnesium oxide smoke escape
* Continue heating until the mass of crucible remains constant (maximum mass), indicating that the reaction is complete
* Measure the mass of crucible and contents (calculate the mass of metal oxide by subtracting the mass of empty crucible)
\
**Working out the empirical formula:**
***Mass of metal:***
Subtract mass of crucible from magnesium and the mass of the empty crucible***Mass of oxygen:***
Subtract mass of the magnesium used from the mass of magnesium oxide
**Step 1 –** Divide each of the two masses by the relative atomic masses of the elements
**Step 2 –** Simplify the ratio
**magnesium** **oxygen**
Mass a b
Mole a / Ar b / Ar
= x = y
Ratio x : y
**Step 3 –** Represent the ratio into the form ‘MxOy‘ E.g, MgO
\
74
New cards
how are ions formed by electron loss or gain
* An ion is an **electrically** **charged** atom or group of atoms formed by the **loss** or **gain** of **electrons**
* This loss or gain of electrons takes place to obtain a **full** **outer** **shell** of electrons
* The electronic structure of ions of elements in groups 1, 2, 3, 5, 6 and 7 will be the same as that of a noble gas - such as helium, neon, and argon
* Negative ions are called **anions** and form when atoms **gain** electrons, meaning they have more electrons than protons
* Positive ions are called **cations** and form when atoms **lose** electrons, meaning they have more protons than electrons
* All metals **lose** electrons to other atoms to become positively charged ions
* All non-metals **gain** electrons from other atoms to become negatively charged ions
\
\
* This loss or gain of electrons takes place to obtain a **full** **outer** **shell** of electrons
* The electronic structure of ions of elements in groups 1, 2, 3, 5, 6 and 7 will be the same as that of a noble gas - such as helium, neon, and argon
* Negative ions are called **anions** and form when atoms **gain** electrons, meaning they have more electrons than protons
* Positive ions are called **cations** and form when atoms **lose** electrons, meaning they have more protons than electrons
* All metals **lose** electrons to other atoms to become positively charged ions
* All non-metals **gain** electrons from other atoms to become negatively charged ions
\
\
75
New cards
the charges of ions:
• metals in Groups 1, 2 and 3
• metals in Groups 1, 2 and 3
Group 1 : 1 +
Group 2 : 2+
Group 3 : 3+
Group 2 : 2+
Group 3 : 3+
76
New cards
the charges of ions:
non-metals in Groups 5, 6 and 7
non-metals in Groups 5, 6 and 7
Group 5 : 3-
Group 6 : 2-
Group 7 : 1-
Group 6 : 2-
Group 7 : 1-
77
New cards
the charges of ions :
Ag, Cu, Fe, Fe, Pb, Zn
Ag, Cu, Fe, Fe, Pb, Zn
Ag+
Cu2+
Fe2+
Fe3+
Pb2+
Zn2+
Cu2+
Fe2+
Fe3+
Pb2+
Zn2+
78
New cards
the charges of :
hydrogen , hydroxide, ammonium, carbonate, nitrate, sulfate
hydrogen , hydroxide, ammonium, carbonate, nitrate, sulfate
hydrogen (H+)
hydroxide (OH–)
ammonium (NH4+)
carbonate (CO32–)
nitrate (NO3-)
sulfate (SO42–)
hydroxide (OH–)
ammonium (NH4+)
carbonate (CO32–)
nitrate (NO3-)
sulfate (SO42–)
79
New cards
write formulae for compounds formed between the ions listed above
* Example: what is the formula of aluminium sulfate?
* Write out the formulae of each ion, including their charges
* Al3+ SO42-
\
* Balance the charges by multiplying them out:
Al3+ x **2** = +6 and SO42- x **3** = -6; so +6 – 6 = 0
* So the formula is Al2(SO4)3
\
\
Another method that also works is to 'swap the numbers'. In the example above the numbers in front of the charges of the ions (3 and 2) are swapped over and become the multipliers in the formula (2 and 3). Easy when you know how!
* Write out the formulae of each ion, including their charges
* Al3+ SO42-
\
* Balance the charges by multiplying them out:
Al3+ x **2** = +6 and SO42- x **3** = -6; so +6 – 6 = 0
* So the formula is Al2(SO4)3
\
\
Another method that also works is to 'swap the numbers'. In the example above the numbers in front of the charges of the ions (3 and 2) are swapped over and become the multipliers in the formula (2 and 3). Easy when you know how!
80
New cards
draw dot-and-cross diagrams to show the formation of ionic compounds by electron
transfer, limited to combinations of elements from Groups 1, 2, 3 and 5, 6, 7
transfer, limited to combinations of elements from Groups 1, 2, 3 and 5, 6, 7
not really sure how to explain dot and cross, find the electronic configuration and the last number in that draw dots/crosses (only one of them) on a circle around the outside of the chemical symbol
81
New cards
what is ionic bonding in terms of electrostatic attractions
* The positive and negative charges are held together by the strong **electrostatic forces of** **attraction** between **oppositely** charged ions
\
***Electrostatic forces hold the ions together***
\
***Electrostatic forces hold the ions together***
82
New cards
why do compounds with giant ionic lattices have high melting and boiling points
* Ionic compounds are made of charged particles called ions which form a giant lattice structure
* Ionic substances have **high** melting and boiling points due to the presence of **stron**g **electrostatic forces** acting between the oppositely charged ions
* These forces act in all directions and a lot of energy is required to overcome them
* Ionic substances have **high** melting and boiling points due to the presence of **stron**g **electrostatic forces** acting between the oppositely charged ions
* These forces act in all directions and a lot of energy is required to overcome them
83
New cards
do ionic compounds conduct electricity when solid ?
* For electrical current to flow there must be present freely moving charged particles such as electrons or ions
* Ionic compounds can conduct electricity in the **molten** state or in **solution** as they have ions that can move and carry charge
* They cannot conduct electricity in the solid state as the ions are in fixed positions within the lattice and are unable to mov
* Ionic compounds can conduct electricity in the **molten** state or in **solution** as they have ions that can move and carry charge
* They cannot conduct electricity in the solid state as the ions are in fixed positions within the lattice and are unable to mov
84
New cards
What is a covalent bond formed by
* Non-metal atoms can share electrons with other non-metal atoms to obtain a **full** **outer** **shell** of electrons
* When atoms share pairs of electrons, they form covalent bonds
* Covalent bonds between atoms are very **strong**
* When two or more atoms are covalently bonded together, they form ‘molecules’
* Covalently bonded substances may consist of small molecules or giant molecules
* Weak intermolecular forces exist between individual molecules
* E.g. Each liquid water molecule consists of two hydrogen atoms covalently bonded to an oxygen atom, and in between two individual water molecules there are weak intermolecular forces
\
* Shared electrons are called **bonding electrons** and occur in **pairs**
* Electrons on the outer shell which are not involved in the covalent bond(s) are called **non-bonding** electrons
* Simple covalent molecules do not conduct electricity as they do not contain free electrons
\
\
\n
* When atoms share pairs of electrons, they form covalent bonds
* Covalent bonds between atoms are very **strong**
* When two or more atoms are covalently bonded together, they form ‘molecules’
* Covalently bonded substances may consist of small molecules or giant molecules
* Weak intermolecular forces exist between individual molecules
* E.g. Each liquid water molecule consists of two hydrogen atoms covalently bonded to an oxygen atom, and in between two individual water molecules there are weak intermolecular forces
\
* Shared electrons are called **bonding electrons** and occur in **pairs**
* Electrons on the outer shell which are not involved in the covalent bond(s) are called **non-bonding** electrons
* Simple covalent molecules do not conduct electricity as they do not contain free electrons
\
\
\n
85
New cards
what is covalent bonds in terms of electrostatic attractions
* In a normal covalent bond, each atom provides one of the electrons in the bond
* A covalent bond is represented by a short straight line between the two atoms, H-H
* Covalent bonds should not be regarded as shared electron pairs in a fixed position; the electrons are in a state of constant motion and are best regarded as **charge clouds**
* Sharing electrons in the covalent bond allows each of the 2 atoms to achieve an electron configuration similar to a noble gas
* This makes each atom more stable
\
\
* A covalent bond is represented by a short straight line between the two atoms, H-H
* Covalent bonds should not be regarded as shared electron pairs in a fixed position; the electrons are in a state of constant motion and are best regarded as **charge clouds**
* Sharing electrons in the covalent bond allows each of the 2 atoms to achieve an electron configuration similar to a noble gas
* This makes each atom more stable
\
\
86
New cards
how do you draw diatomic dot and cross diagrams
draw two dot and cross diagrams overlapping and have a full outershell
\
you can do this with as many circles as needed
\
you can do this with as many circles as needed
87
New cards
explain why substances with a simple molecular structures are gases or liquids, or solids with low melting and boiling points
* Simple molecular structures have covalent bonds joining the atoms together, but intermolecular forces that act between neighbouring molecules
* They have **low** melting and boiling points as there are only **weak intermolecular** forces acting **between** the molecules
* These forces are **very weak** when compared to the covalent bonds and so most small molecules are either gases or liquids at room temperature
* Often the liquids are volatile
\
* As the molecules increase in **size** the intermolecular forces also increase as there are more electrons available
* This causes the melting and boiling points to **increase**
\
the term intermolecular forces of attraction can be used to represent all forces between molecules
* They have **low** melting and boiling points as there are only **weak intermolecular** forces acting **between** the molecules
* These forces are **very weak** when compared to the covalent bonds and so most small molecules are either gases or liquids at room temperature
* Often the liquids are volatile
\
* As the molecules increase in **size** the intermolecular forces also increase as there are more electrons available
* This causes the melting and boiling points to **increase**
\
the term intermolecular forces of attraction can be used to represent all forces between molecules
88
New cards
explain why the melting and boiling points of substances with simple molecular structures increase, in general, with increasing relative molecular mass
* As the relative molecular mass of a substance increases, the melting and boiling point will increase as well
* An increase in the relative molecular mass of a substance means that there are more electrons in the structure, so there are more intermolecular forces of attraction that need to be overcome when a substance changes state
* So larger amounts of heat energy are needed to overcome these forces, causing the compound to have a higher melting and boiling point
* The family of organic molecules called alkanes show a clear increase in boiling point as the size of the molecule increases
* An increase in the relative molecular mass of a substance means that there are more electrons in the structure, so there are more intermolecular forces of attraction that need to be overcome when a substance changes state
* So larger amounts of heat energy are needed to overcome these forces, causing the compound to have a higher melting and boiling point
* The family of organic molecules called alkanes show a clear increase in boiling point as the size of the molecule increases
89
New cards
explain why substances with giant covalent structures are solids with high melting and boiling points
explain why substances with giant covalent structures are solids with high melting and boiling points
90
New cards
explain how the structures of diamond, influence their
physical properties, including electrical conductivity and hardness
physical properties, including electrical conductivity and hardness
* Diamond and graphite are allotropes of carbon
* Both substances contain only carbon atoms but due to the differences in bonding arrangements they are physically completely different
* In diamond, each carbon atom bonds with four other carbons, forming a **tetrahedron**
* All the covalent bonds are identical, very strong and there are no **intermolecular forces**
\
\
* Diamond has the following physical properties:
* It does not conduct electricity
* It has a very high melting point
* It is extremely hard and has a density of 3.51 g / cm3 – a little higher than that of aluminium
\
* All the outer shell electrons in carbon are held in the four covalent bonds around each carbon atom, so there are no freely moving charged particles to the current
* The four covalent bonds are very strong and extend in a giant lattice, so a very large amount of heat energy is needed to break the lattice
* Diamond ́s hardness makes it very useful for purposes where extremely tough material is required
* Diamond is used in **jewellery** and for coating blades in **cutting tools**
* The cutting edges of discs used to cut bricks and concrete are tipped with diamonds
* Heavy-duty drill bits and tooling equipment are also diamond tipped
* Both substances contain only carbon atoms but due to the differences in bonding arrangements they are physically completely different
* In diamond, each carbon atom bonds with four other carbons, forming a **tetrahedron**
* All the covalent bonds are identical, very strong and there are no **intermolecular forces**
\
\
* Diamond has the following physical properties:
* It does not conduct electricity
* It has a very high melting point
* It is extremely hard and has a density of 3.51 g / cm3 – a little higher than that of aluminium
\
* All the outer shell electrons in carbon are held in the four covalent bonds around each carbon atom, so there are no freely moving charged particles to the current
* The four covalent bonds are very strong and extend in a giant lattice, so a very large amount of heat energy is needed to break the lattice
* Diamond ́s hardness makes it very useful for purposes where extremely tough material is required
* Diamond is used in **jewellery** and for coating blades in **cutting tools**
* The cutting edges of discs used to cut bricks and concrete are tipped with diamonds
* Heavy-duty drill bits and tooling equipment are also diamond tipped
91
New cards
explain how the structures of graphite influence their physical properties, including electrical conductivity and hardness
* Each carbon atom in graphite is bonded to **three** others forming **layers** of **hexagons**, leaving one free electron per carbon atom
* These free electrons migrate along the layers and are free to move and carry charge, hence graphite can **conduct electricity**
* The covalent bonds within the layers are very strong, but the layers are attracted to each other by weak **intermolecular forces**, so the layers can **slide** over each other making graphite **soft** and **slippery**
\
**Properties of Graphite**
* Graphite has the following physical properties:
* It conducts electricity and heat
* It has a very high melting point
* It is soft and slippery and less dense than diamond (2.25 g / cm3)
\
* The weak intermolecular forces make it a useful material
* It is used in **pencils** and as an industrial **lubricant**, in engines and in locks
* It is also used to make **inert** **electrodes** for **electrolysis**, which is particularly important in the extraction of metals such as aluminium
\
\
* These free electrons migrate along the layers and are free to move and carry charge, hence graphite can **conduct electricity**
* The covalent bonds within the layers are very strong, but the layers are attracted to each other by weak **intermolecular forces**, so the layers can **slide** over each other making graphite **soft** and **slippery**
\
**Properties of Graphite**
* Graphite has the following physical properties:
* It conducts electricity and heat
* It has a very high melting point
* It is soft and slippery and less dense than diamond (2.25 g / cm3)
\
* The weak intermolecular forces make it a useful material
* It is used in **pencils** and as an industrial **lubricant**, in engines and in locks
* It is also used to make **inert** **electrodes** for **electrolysis**, which is particularly important in the extraction of metals such as aluminium
\
\
92
New cards
explain how the structures of C60 fullerene influence their physical properties, including electrical conductivity and hardness
* Fullerenes are a group of carbon allotropes which consist of molecules that form **hollow tubes** or **spheres**
* Fullerenes can be used to trap other molecules by forming around the target molecule and capturing it, making them useful for targeted **drug delivery** systems
* They also have a **huge surface area** and are useful for trapping **catalyst** molecules onto their surfaces making them easily accessible to reactants so catalysis can take place
* Some fullerenes are excellent lubricants and are starting to be used in many industrial processes
* The first fullerene to be discovered was buckminsterfullerene which is affectionately referred to as a “buckyball”
* In this fullerene, 60 carbon atoms are joined together forming 20 hexagons and 12 pentagons which produce a hollow sphere that is the exact shape of a soccer ball
\
\
\
* Fullerenes can be used to trap other molecules by forming around the target molecule and capturing it, making them useful for targeted **drug delivery** systems
* They also have a **huge surface area** and are useful for trapping **catalyst** molecules onto their surfaces making them easily accessible to reactants so catalysis can take place
* Some fullerenes are excellent lubricants and are starting to be used in many industrial processes
* The first fullerene to be discovered was buckminsterfullerene which is affectionately referred to as a “buckyball”
* In this fullerene, 60 carbon atoms are joined together forming 20 hexagons and 12 pentagons which produce a hollow sphere that is the exact shape of a soccer ball
\
\
\
93
New cards
Do covalent compounds usually conduct electricity
no not usually
94
New cards
TRIPLE: know how to represent a metallic lattice by a 2-D diagram
diagram is circles with + signs and - signs randomly
95
New cards
TRIPLE: explain metallic bonding in terms of electrostatic attractions
Metallic bonding is the strong electrostatic force of attraction between the metal ions and the delocalised electrons.
96
New cards
TRIPLE:explain typical physical properties of metals, including electrical conductivity
and malleability
and malleability
* Metals have **high** melting and boiling points
* There are many **strong** **metallic** **bonds** in giant metallic structures
* A lot of heat energy is needed to overcome forces and break these bonds
\
* Metals **conduct** electricity
* There are **free** **electrons** available to move and carry charge
* Electrons entering one end of the metal cause a delocalised electron to displace itself from the other end
* Hence electrons can flow so electricity is conducted
\
* Metals are **malleable** and **ductile**
* Layers of positive ions can **slide** over one another and take up different positions
* Metallic bonding is not disrupted as the valence electrons do not belong to any particular metal atom so the delocalised electrons will move with them
* Metallic bonds are thus not broken and as a result metals are strong but **flexible**
* They can be hammered and bent into different shapes without breaking
* There are many **strong** **metallic** **bonds** in giant metallic structures
* A lot of heat energy is needed to overcome forces and break these bonds
\
* Metals **conduct** electricity
* There are **free** **electrons** available to move and carry charge
* Electrons entering one end of the metal cause a delocalised electron to displace itself from the other end
* Hence electrons can flow so electricity is conducted
\
* Metals are **malleable** and **ductile**
* Layers of positive ions can **slide** over one another and take up different positions
* Metallic bonding is not disrupted as the valence electrons do not belong to any particular metal atom so the delocalised electrons will move with them
* Metallic bonds are thus not broken and as a result metals are strong but **flexible**
* They can be hammered and bent into different shapes without breaking
97
New cards
TRIPLE:
why don’t covalent compounds conduct electricity
why don’t covalent compounds conduct electricity
* Most covalent compounds do not conduct electricity as they have no freely moving charged particles to carry the current
98
New cards
TRIPLE : why do ionic compounds conduct electricity only when molten or in aqueous solution
* Ionic compounds can conduct electricity in the **molten** state or in **solution** as they have ions that can move and carry charge
* They cannot conduct electricity in the solid state as the ions are in fixed positions within the lattice and are unable to move
* They cannot conduct electricity in the solid state as the ions are in fixed positions within the lattice and are unable to move
99
New cards
what is an anode
* Negative ions within the electrolyte migrate towards the **positively** charged electrode which is the **anode**
100
New cards
what is a cathode
* Positive ions within the electrolyte migrate towards the **negatively** charged electrode which is the **cathode**