CMMB 343 Midterm 1 Flashcards
1/164
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
165 Terms
Discover of Cells/Bacteria History
Who discovered cells ?
Robert Hooke examined cork cells and coined the term cell in the 1600’s.
Who discovered bacteria?
Antoni van Leeuwenhoek examined pond water and human fluids under higher magnification and was the first to observe bacteria, reported his observations of “wee bacteria” in the 1600’s.
Louis Pasteur Discovery of Microbial Metabolism
First to discover that chemical reactions were metabolic reactions of microbes.
Alcohol fermentation: Sugars → Ethanol
While investigating fermentation of beet juice lactic acid was being produced instead of ethanol he observed:
healthy vats contained ethanol producing yeast.
spoiled vats contained lactate producing bacteria.
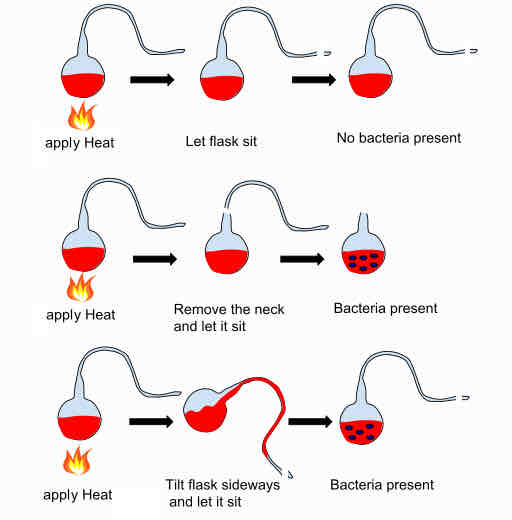
Steps of the Swan-Necked Flask
1) Addition of liquid medium containing nutrients into flask.
2) Using a flame sterilize medium and elongate the neck of the flask to include a swan bend.
3) Dust/microbes get trapped in bend, medium remains sterile.
4) If flask tipped the liquid spoils.
Louis Pasteur Developments
Sterilization
Aseptic Technique
Pasteurization
Vaccines
Using attenuated (old) strains made crude vaccines for anthrax and rabies.
Contributions of Robert Koch
Germ Theory of Disease
Demonstrated link between microbes and infectious disease.
Introduced Koch’s Postulates
Koch’s Postulates (Major Steps: 4)
Criteria for associating a microbe with a disease.
Suspected pathogen must be present in all diseased animals and absent in health individuals.
Suspected pathogen must be isolated and grown in a pure culture.
Cells from a pure culture of pathogen must be able to infect a healthy individual.
The pathogen must be isolated and cultured again and shown to be the same pathogen as the original.
Culture Techniques
Robert Koch developed multiple growing methods for microbes and developed staining techniques.
Walther Hesse and Richard Petri developed mechanism to grow microbes on solid media.
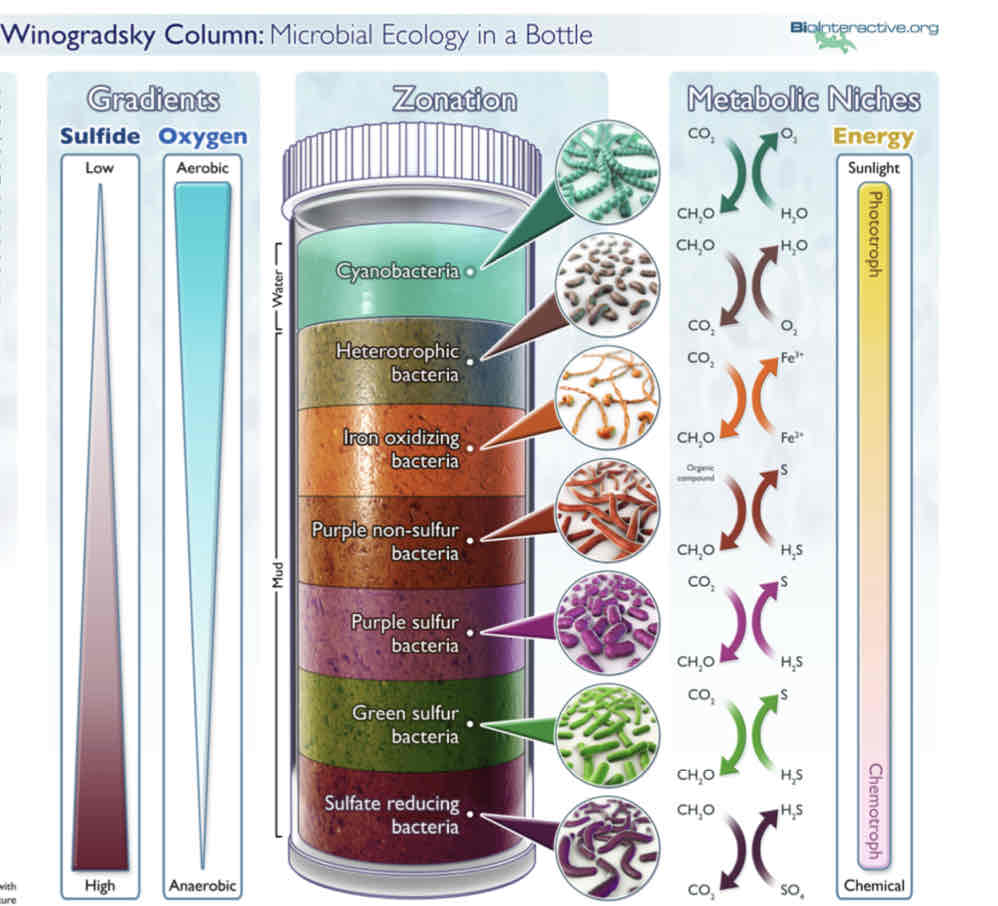
Sergei Winogradsky and Microbial Ecology
Demonstrated that specific bacteria associated with specific biogeochemical transformations.
Nitrogen and Sulfur Cycles
Sergei Winogradsky and Discoveries (3 Major)
Described :
Chemolithotrophy (oxidation of inorganic molecules for energy)
Autotrophy (CO2 as carbon source)
Microbial Nitrogen Fixation (N2→NH2) and nitrification ( NH3 → NO2 → NO3)
Winogradsky Column (Gradients of Sulfide and Oxygen and Metabolic Niches)
Martinus Beijerinck and Enrichment Culture
Developed multiple techniques to select for organisms with specific metabolic requirements.
Martinus Beijerinck Major Discoveries (2)
Discovered symbiotic nitrogen fixing bacteria is root nodules of legumes.
First to isolate virus
4 Ways to Study Diversity of Microbes
Morphological Diversity (look like, made of)
Metabolic Diversity (source of carbon / energy)
Genomic Diversity (varying functions of microbes)
Evolutionary Diversity (placement on tree of life)
Who is Ferdinand Cohn and contributions?
Considers father of taxonomy.
Coined the term bacteria.
Organized microbes into 4 groups based on cell shape.
What are sphere shaped bacteria called?
Sphaerobacteria (micrococcus)
What are microbacteria (bacterium)
Rod-shaped
What bacteria are filamentous
Desmobacterium (Bacillus, Vibrio)
What bacteria are coiled
Spirobacterium ( Spirillum, Spirochaete)
Metabolic Diversity: Energy and Carbon Sources
Energy:
Chemolithotrophy : Inorganic molecules
Chemoorganotrophy: Organic Molecules
Carbon:
Heterotrophy: organic molecules
Autotrophy: CO2
Metabolic Diversity Characteristics (4)
O2 requirements : aerobic, anaerobic, facultative
Growth conditions (temp, pH, etc.)
Nutrient Requirements
Relationships with other organisms (planktonic, symbiotic, parasitic)
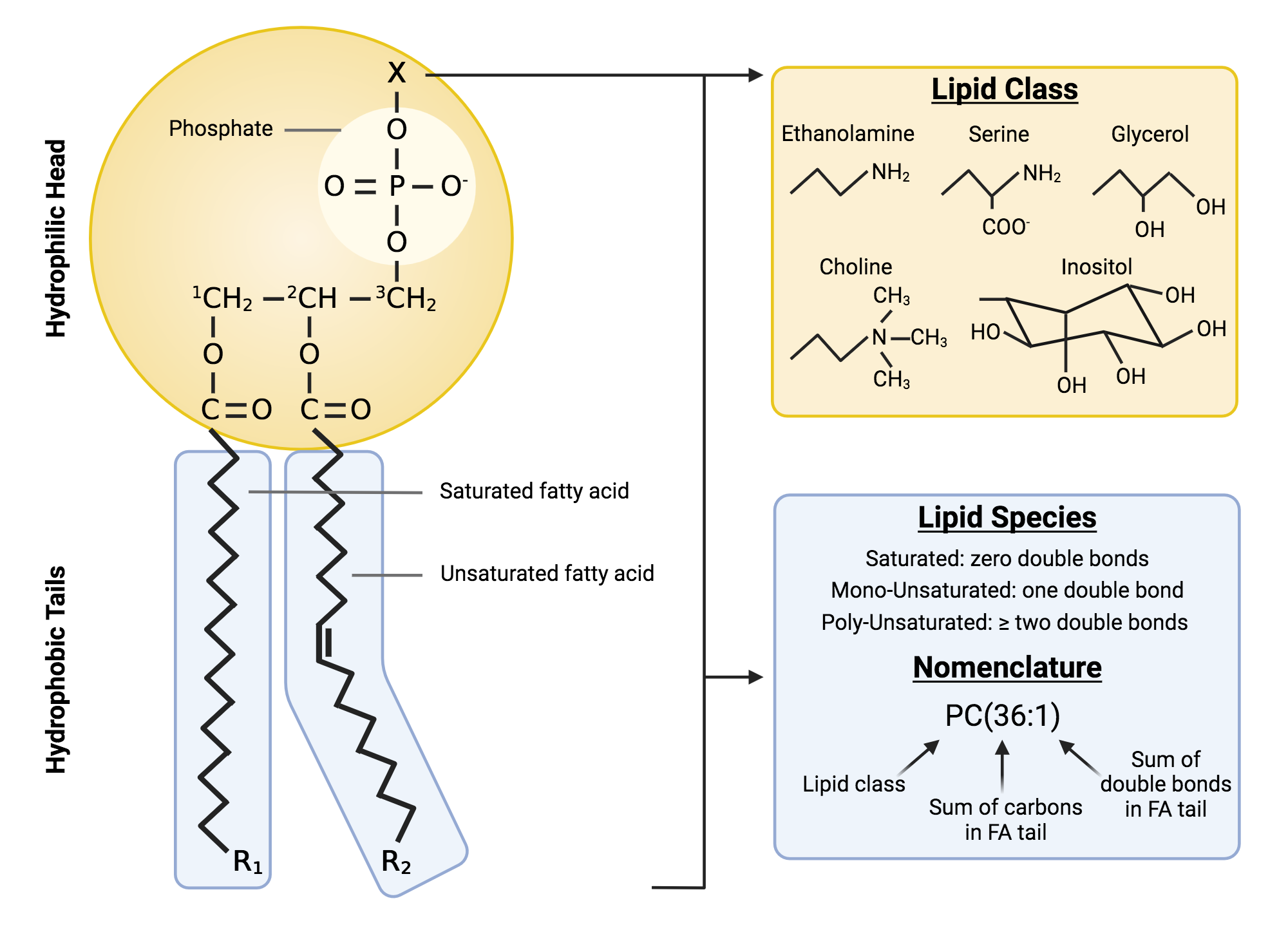
Bacterial Cytoplasmic Membrane
General Structure: Phospholipid Bilayer containing embedded proteins
Contain:
Hydrophobic fatty acid tails (about 12-20C long)
Hydrophilic glycerol+phosphate+functional group.
membrane permeability:
Non-polar molecules and small weakly polar molecules (H2O, CH4,O2) will pass through.
Membrane does not allow passage of large molecules (sugars, aa)
Adaptations of lipid membranes in thermophiles
Bacteria can change saturation of fatty acid tails based on temperature.
In thermophiles high saturation compensates for high temperatures. Tighter packing decreases fluidity.
Archael Membranes
Whereas bacteria/eukarya have ester linkages in phospholipids archaea have ether (C-O-C) linkages.
Archael lipids lack fatty acids have isoprenes instead.
Major lipids include : glycerol diethers with 20C phytanyl side chains and diglycerol tetraethers with 40C biphytanyl side chains.
Archaeal membranes exist as mono layers or bilayers or both.
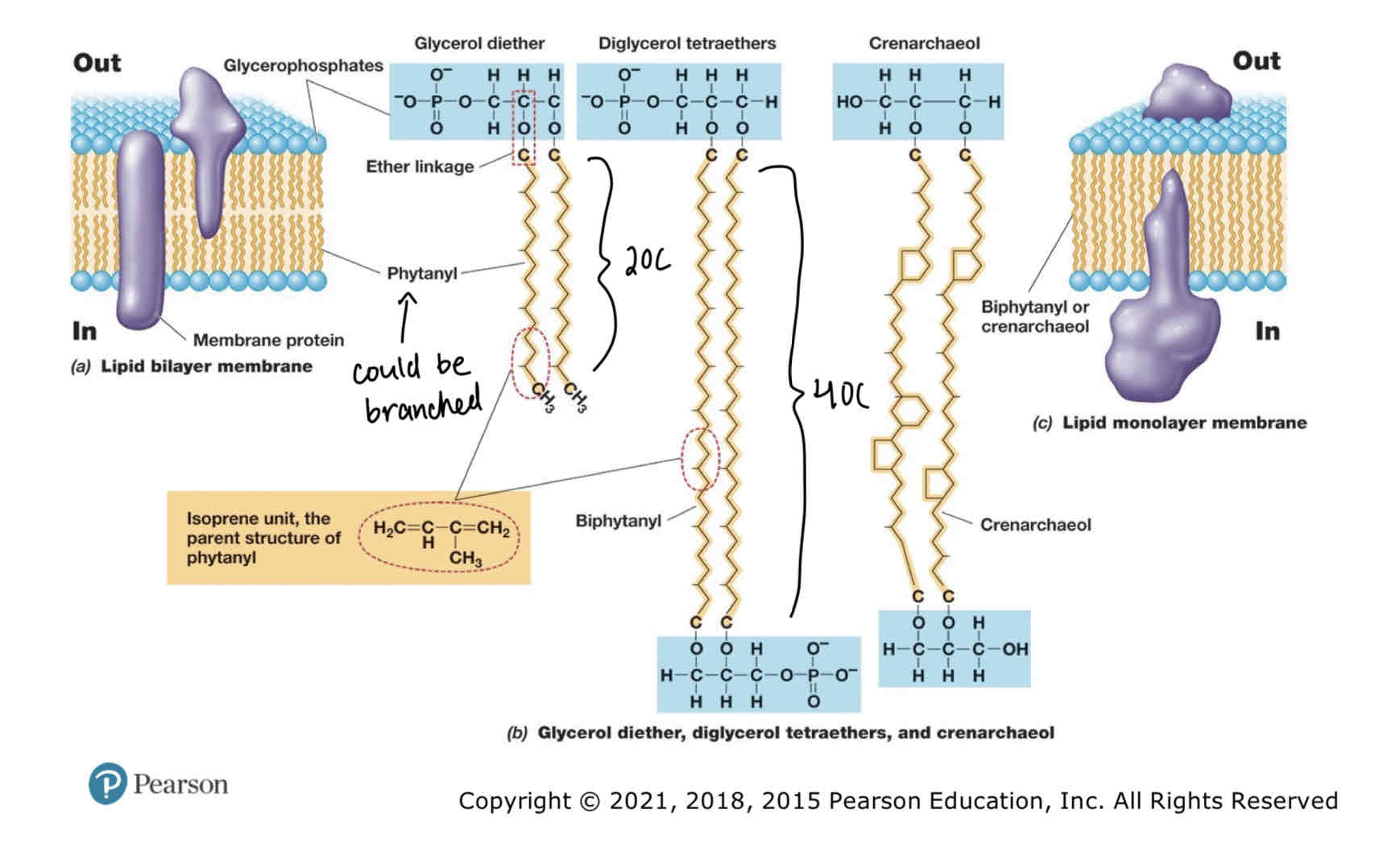
Archaea Bylayer Lipids Structure
Composed of glycerol diether:
Hydrophilic head consists of glycerophosphates and glycerol backbone.
20C Hydrophobic phytanyl tails linked via ester linkages. Phytanyl made of isoprene subunits.
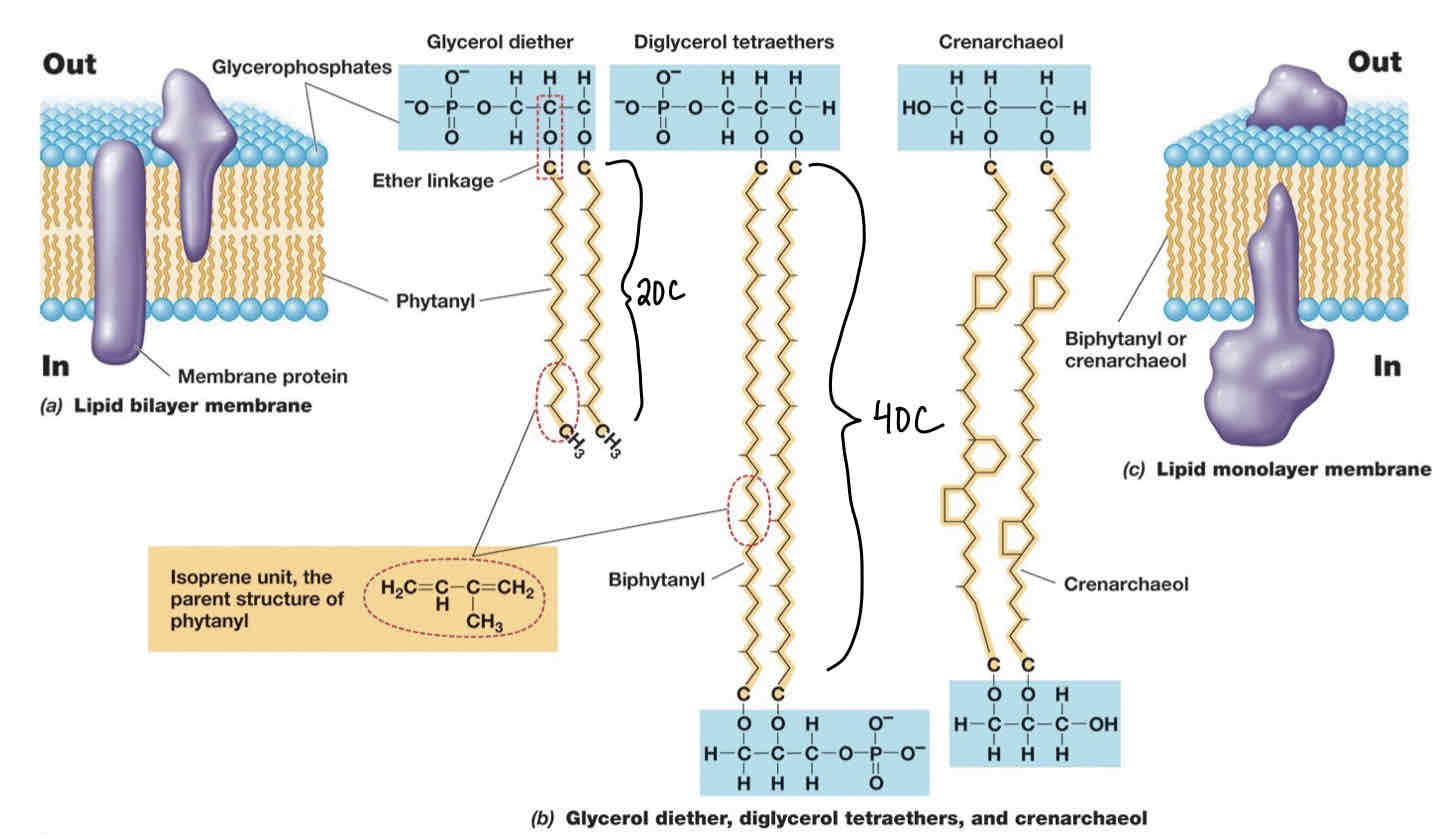
Archaeal Monolayer Major Lipid Structure
Consists of Diglycerol tetraethers:
Same subunits as glycerol diethers.
However: 4 ether linkages , 2 per glycerol backbone (hence diglycerol).
Crenarchaeol are another lipid that can be used in membranes.
Consist of crenarchaeol subunits and OH groups instead of phosphates attached to glycerol backbones.
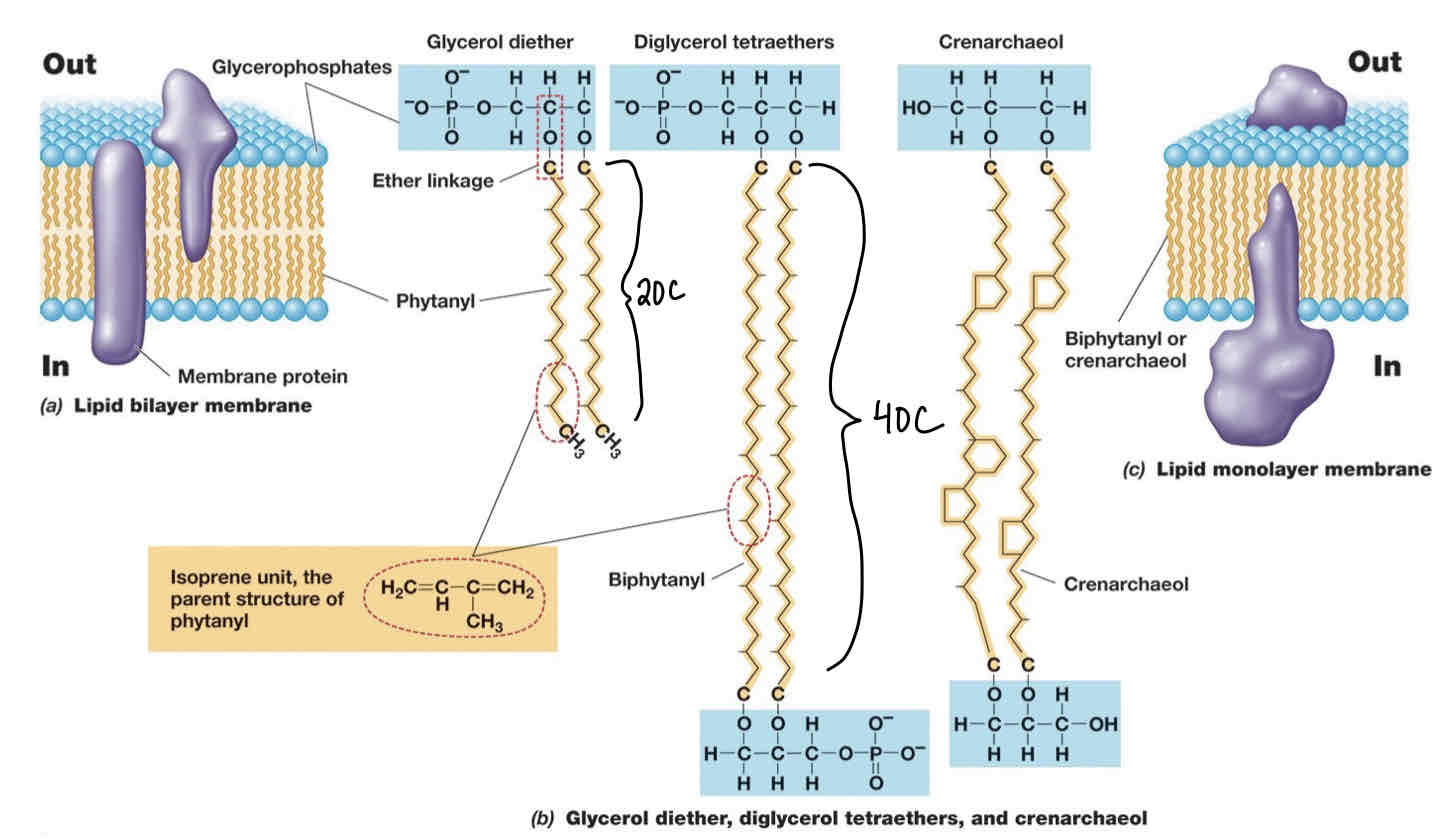
Archaeal Membranes and adaptions of thermophiles (2 mechanisms)
Ether linkages stronger than ester and can withstand higher temperatures.
Archaeal lipids can exist in monolayers (aids in decreasing membrane fluidity).
Major Functions of the Cytoplasmic Membrane (3)
1) Permeability Barrier: Prevents leakages and controls what goes into/out of the cells.
2) Proteins Anchor: site of proteins that participate in transport, bio energetics and chemotaxis.
3) Energy Conservation : Site of generation/maintenance of proton motive force.
Membrane Proteins Types (3)
Integral membrane proteins: embedded proteins
Transmembrane proteins: extend across membrane.
Peripheral Membrane Proteins: Loosely attached.
Active Transport transporters (two mechanisms)
Simple Transport: Involves transmembrane transport proteins and proton motive force, can be symporter or antiporter.
Group Translocation : Series of proteins transport using chemical modification of transported substrate using energy (ATP or PEP).
Cell Envelope Structures (3)
Cell Membrane (1 (gram +) or 2 (gram -))
Cell Wall
S-layers
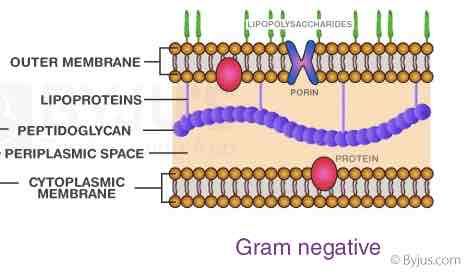
Gram Negative Cell Envelope Arrangements:
Cytoplasmic Membrane
Peptidoglycan Thin cell wall
Periplasm
Outer Membrane (Cell Wall) (lipopolysaccharides and proteins)
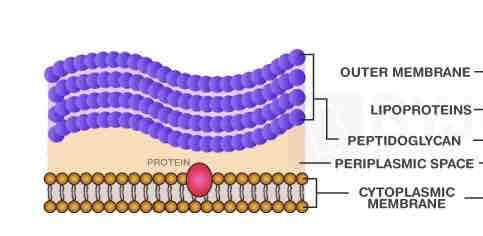
Gram Positive Cell Envelope Arrangements:
Cytoplasmic Membrane
Periplasmic Space
Thick Cell Wall (peptidoglycan)
Which bacteria are +/-
Most bacteria are gram negative, Firmicutes, actinobacteria and some chloroflexi are gram positive,
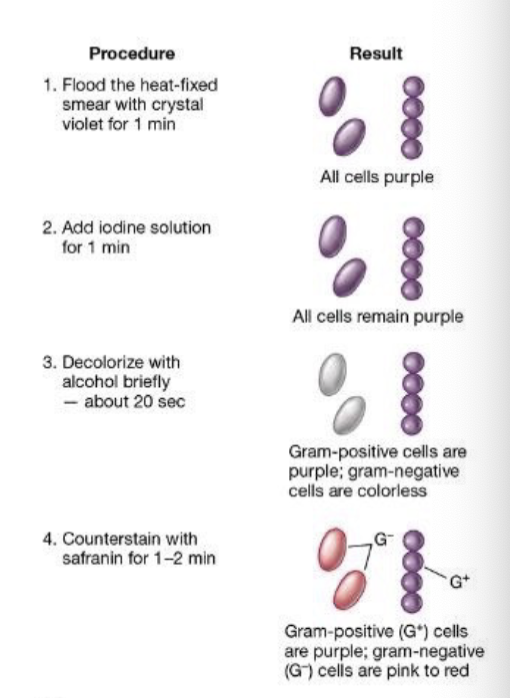
Staining technique for Bacteria
Crystal Violet Application
Both Gram-positive and Gram-negative bacteria take up the primary stain, crystal violet, turning them purple.
Iodine Treatment (Mordant)
Iodine forms a complex with crystal violet, making it more difficult to wash out.
Alcohol/Acetone Wash (Decolorization Step)
Gram-positive bacteria:
Have a thick peptidoglycan layer that retains the crystal violet-iodine complex, keeping them purple.
Gram-negative bacteria:
Have a thin peptidoglycan layer and an outer membrane.
The alcohol wash disrupts the outer membrane and washes out the crystal violet, making them colorless.
Safranin Counterstain
Since Gram-negative bacteria lost their purple color, they take up safranin, turning them pink/red.
Gram-positive bacteria already retain the purple stain, so they do not take up the safranin as visibly.
Thus, after the full Gram staining process:
Gram-positive bacteria remain purple.
Gram-negative bacteria appear pink/red due to safranin.
Functions of the Cell Wall (2)
Maintains cell shape and rigidity
Prevents osmotic lysis and protects against environmental stress.
What are bacterial cell walls made of?
Peptidoglycan : rigid polysaccharide layer that provides structural support and protection to bacterial cells, not found in archaea or eukarya.
Structure of Glycan Tetrapeptide:
Contains:
Sugar backbone of alternating modified glucoses N-acetylglucosamine (NAG or G) and N-acetylmuramic acid (NAM or M).
4 amino acids in chain attached to M. (Amino acids can vary)
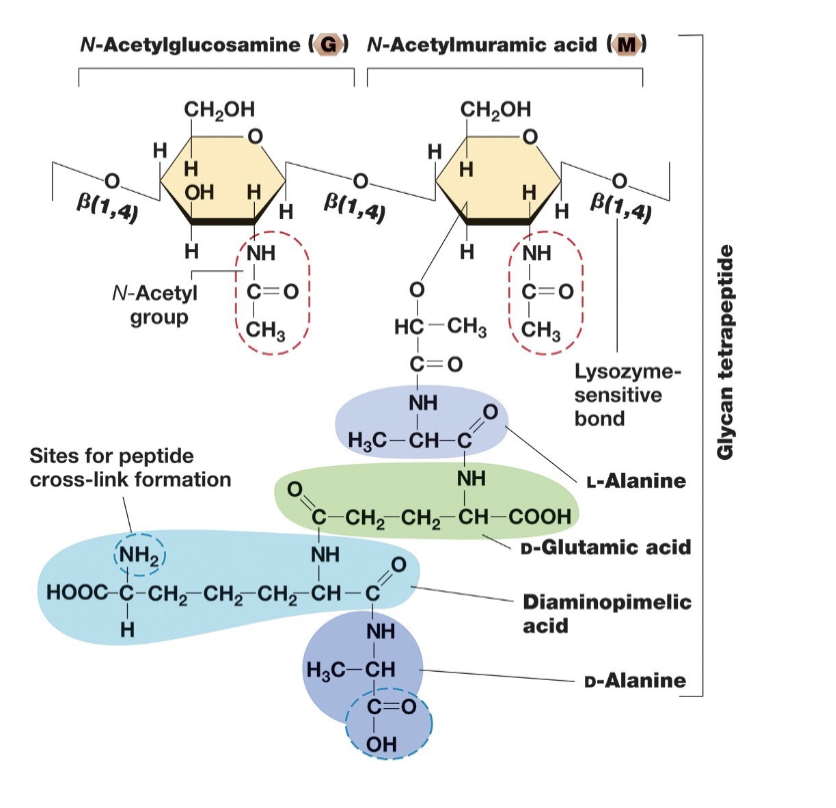
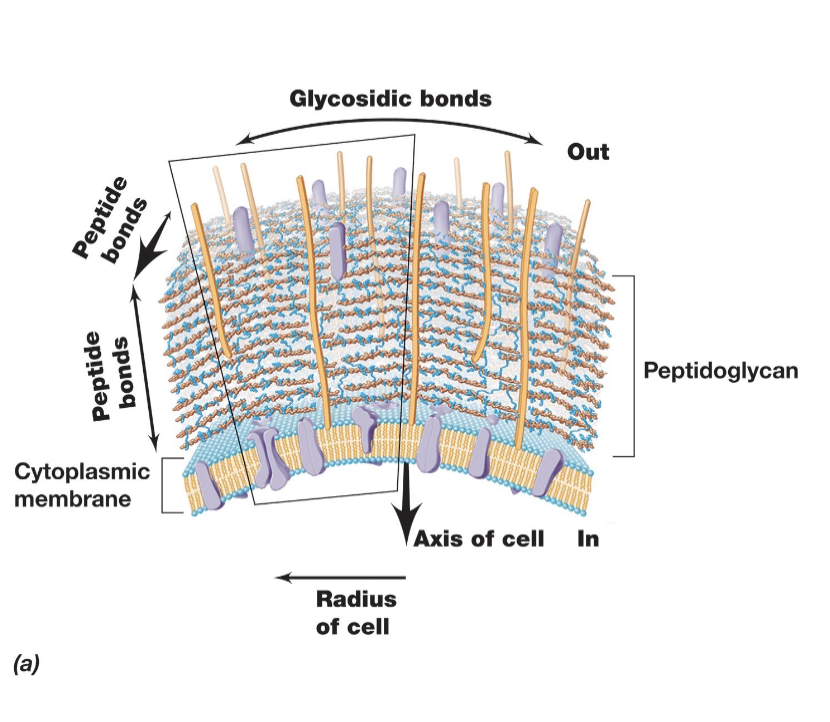
In Bacterial Cell Walls Peptidoglycan (Directionality and Gram +/- linkages):
Strands run parallel around the cell circumference and are cross-linked by covalently bonded peptide chains, providing strength and stability to the cell wall.
In gram negative bacteria linkages are between DAP and D-alanine on adjacent glycan strands.
In gram positive crosslinks usually involve peptide interbridges (eg. 5 glycines connecting the strands, which can lead to thicker walls).
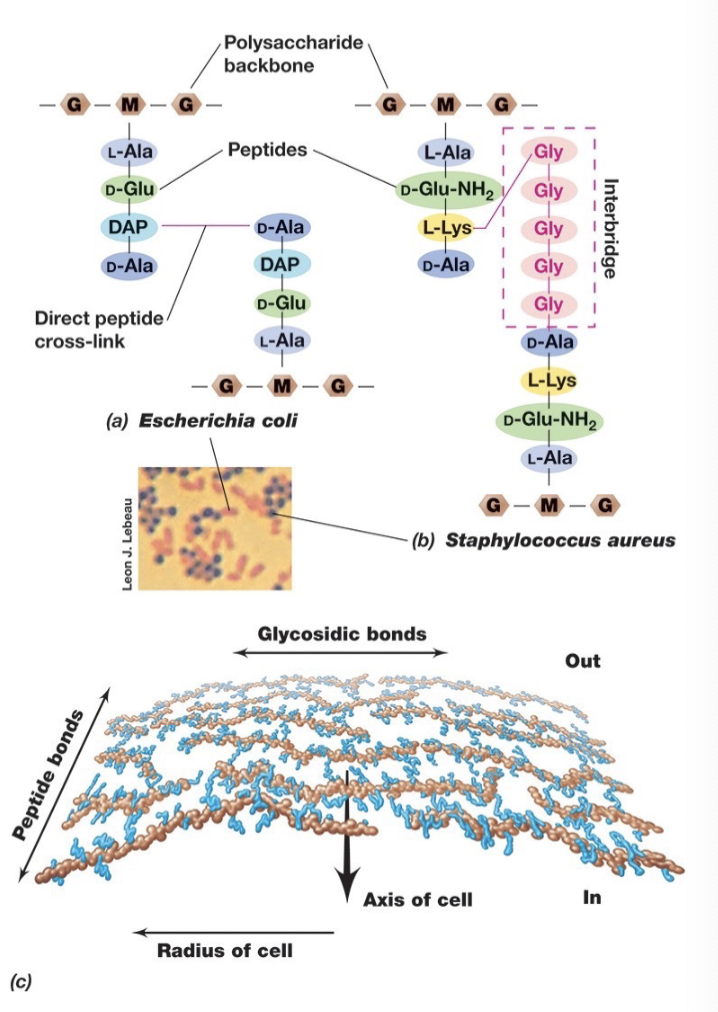
Key Components of Peptidoglycan:
Polysaccharide Backbone – Consists of alternating N-acetylglucosamine (NAG or G) and N-acetylmuramic acid (NAM or M) sugars.
Tetrapeptide Chain – Attached to the NAM (M) sugar, consisting of four amino acids.
Peptide Cross-Linking – Provides structural integrity by linking tetrapeptide chains from different glycan strands.
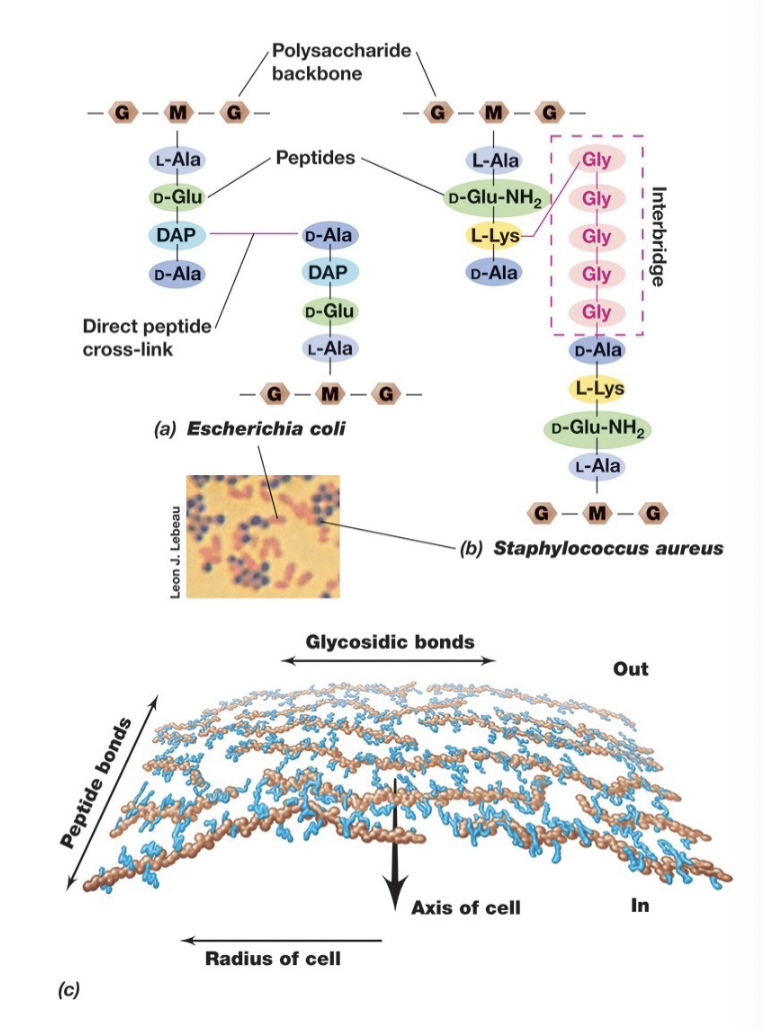
Gram Positive Cell Walls
Gram-positive cell walls can contain up to 90% peptidoglycan, 15
or more layers thick
Gram negatives often have only a single layer
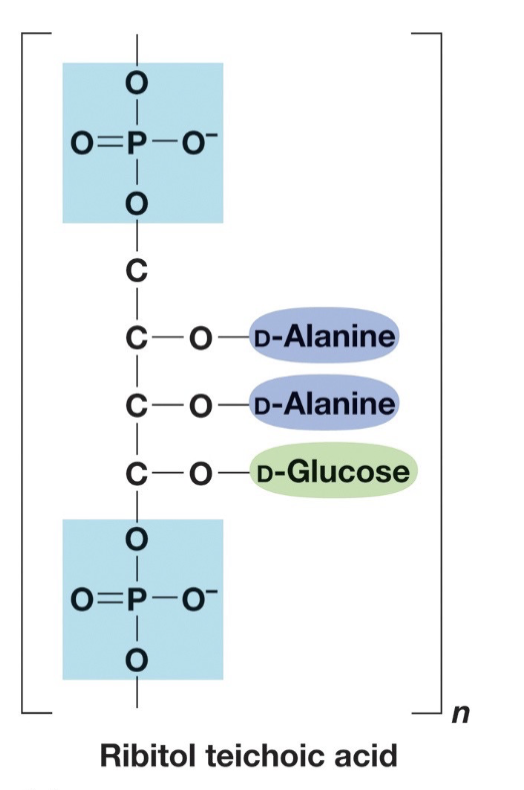
Contain teichoic acids
Teichoic Acid
Acidic, found in some Gram + not in gram -
Strongly antigenic (can initiate immune response)
Covalently bonded to peptidoglycan.
Lipoteichoic acids: teichoic
acids also covalently
bound to membrane lipids.
Structure of Gram Positive Bacterial Cell Walls (4 Major)
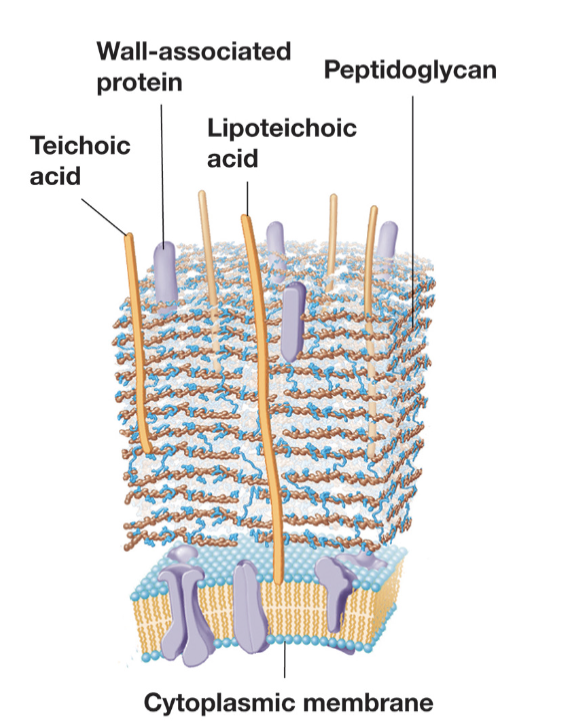
Functions of Teichoic Acid (5)
Maintains porous cell wall.
Anchors cell wall to membrane.
Maintains cell shape.
Capture cations (Ca2+, Mg2+)
Reservoir of phosphate.
Gram Negative Cell Envelope Key Structures
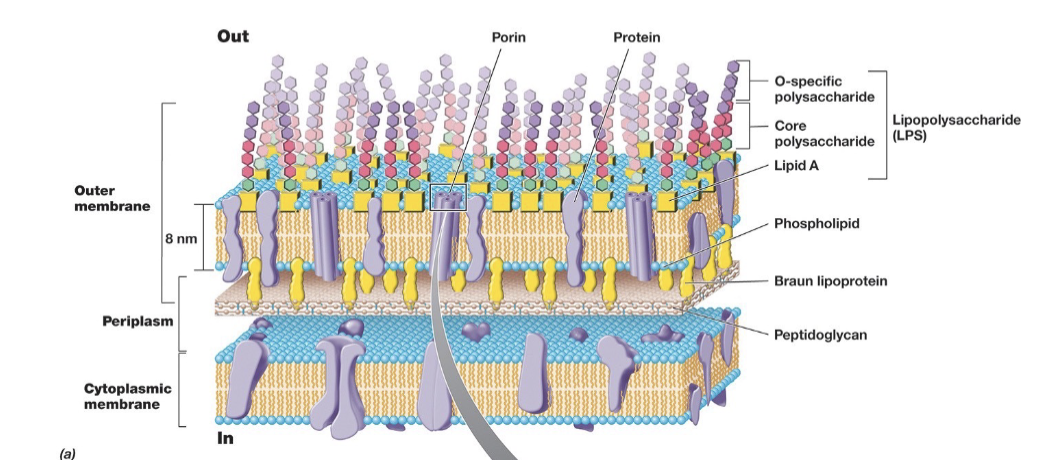
Features of G- Cell Envelope: Periplasm
Space located between cytoplasmic and outer membranes.
About 15nm wide.
Includes many proteins.
Features of G- Cell Envelope: Porins
Channels for movement of hydrophilic small molecules through outer membrane.
Features of G- Cell Envelope: Lipopolysaccharide
Instead of phospholipids, LPS is found on the outer half of the outer membrane.
Consist of polysaccharide covalently bonded to lipids.
Facilitates: Surface recognition, important virulence factors, and gives strength to wall.
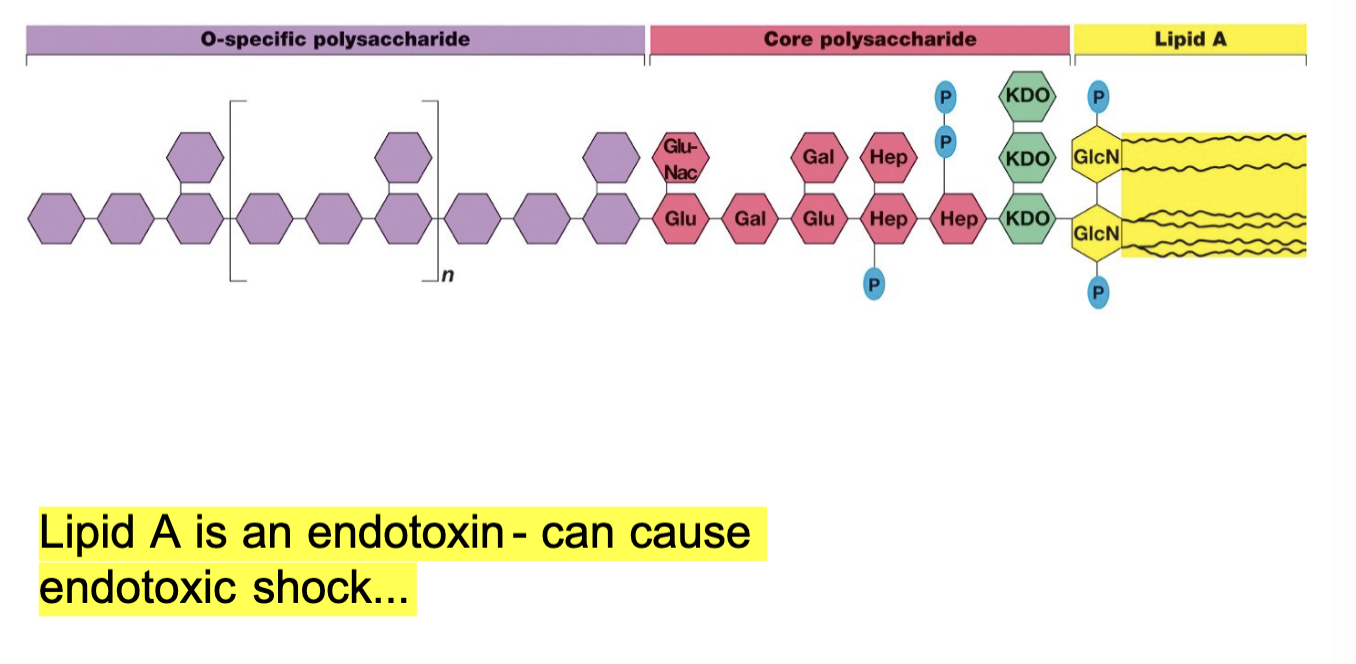
Structure of Bacterial Lipopolysaccharide
3 Main Parts:
Lipid A
Core Polysaccharide
O-specific polysaccharide
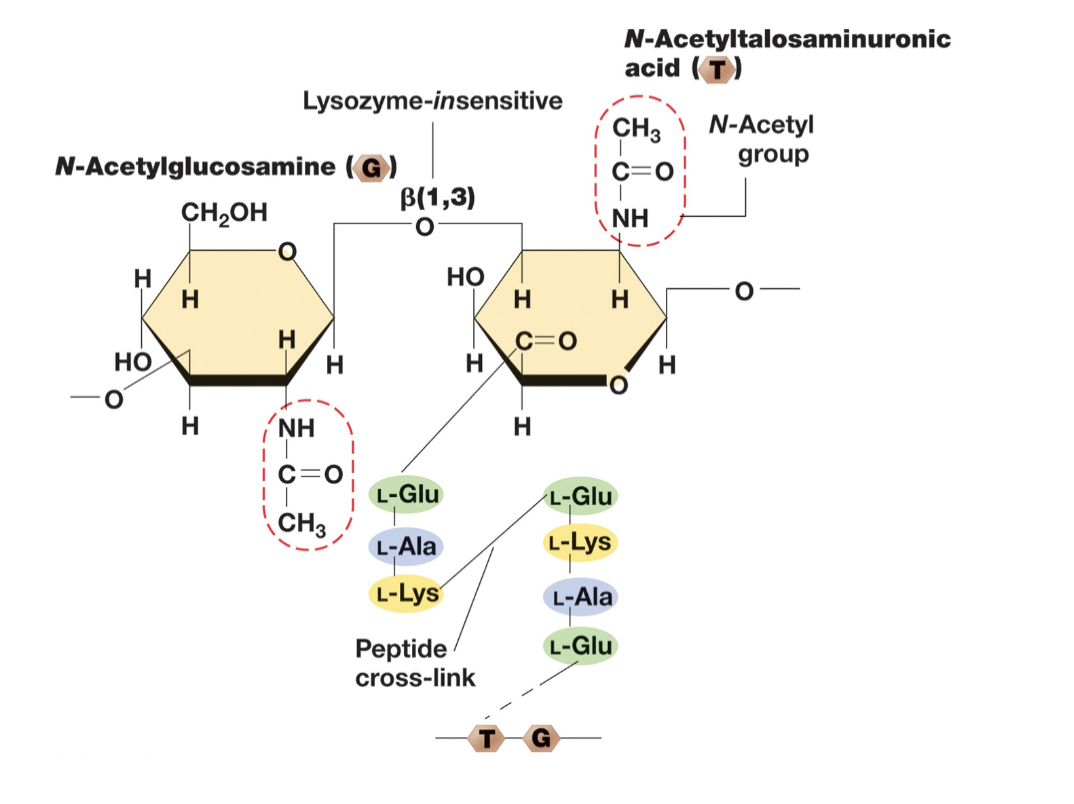
Pseudomurein in Archael Cell Walls:
Pseudomurein in archaea is an analog to the peptidoglycan (glycan tetrapeptide) found in bacteria.
Some differneces:
Instead of NAG-NAM sugar composition it is NAG-NAT.
Contains β-1,3 linkages (resistant to lysozyme) instead of β-1,4 linkage (targeted by lysozyme)
DAP is absent
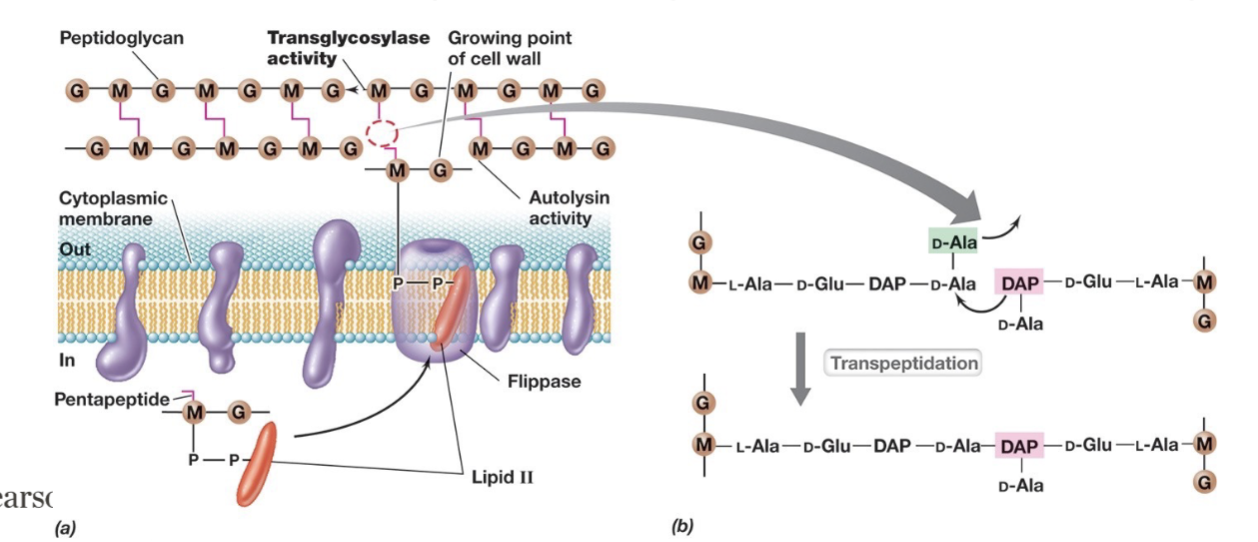
Steps in Peptidoglycan Synthesis (4 steps)
Autolysins break the β-1,4 bonds between G-M sugars in peptidoglycan chains.
Lipid ll is assembled and transported across the membrane via flippase.
Transglycosylases help rebuild the peptidoglycan structure, by polymerizing the glycan backbone by catalyzing the formation of β-1,4 glycosidic bonds between NAG and NAM. (this can be inhibited via vancomycin).
Transpeptidation : enzyme-catalyzed reaction that forms peptide cross-links between peptidoglycan chains.
Agents that Destroy Peptidoglycan:
Lysozyme:
Found in tears, egg whites.
Hydrolyses peptidoglycan by breaking the β-1-4 bond between M-G sugars .
Antibiotics
eg. Vancomysin, β-lactam antibiotic (penicillin)
Inhibit formation and/or cross linking of glycan strands.
Bacteria quickly figure out how to outsmart antibiotics via enzymatic degradation (e.g. Beta- lactamases) and membrane alterations, leads to bacterial resistance.
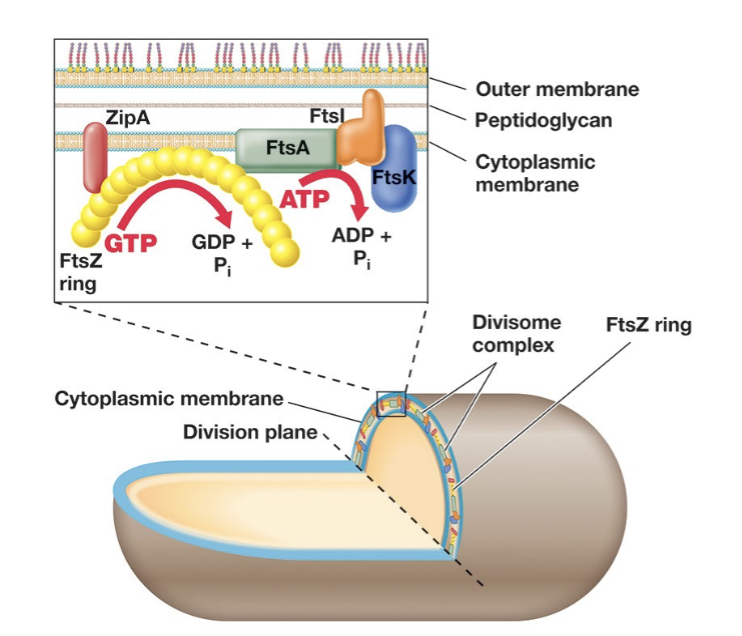
Fts Proteins (5 Major Proteins) and Cell Division:
Prokaryote membranes are involved in replication.
Fts (filamentous-temperature-sensitive proteins) are essential in cell division in all prokaryotes.
Interact to form the divisome protein complex.
FtsZ : forms ring around the centre of the cell, structure homologous to tubulin in eukaryotes.
ZipA: anchor that connects FtsZ ring to cytoplasmic membrane.
FtsI is involved in peptidoglycan synthesis, FtsK aids in chromosome separation, and FtsA is an ATPase that helps regulate the process.
Spatial Regulation of Cell Division (Min System)
MinCD prevents FtsZ ring formation.
MinE moves between poles, pushing MinCD aside.
As the cell elongates, MinCD concentration is low in the center, allowing FtsZ ring formation.
Determinants of Cell Morphology: Crescentin (Function and Role):
Shape-determining protein found in curved cells:
• organizes into filaments ~10 nm wide that localize on concave face of the curved cells
Determinants of Cell Morphology: MreB (Function and Role):
MreB is a key shape-determining protein in prokaryotes.
Forms a cytoskeleton with patchlike filaments beneath the membrane.
Absent in coccus-shaped (spherical) bacteria.
Recruits proteins to guide cell wall growth in a specific pattern
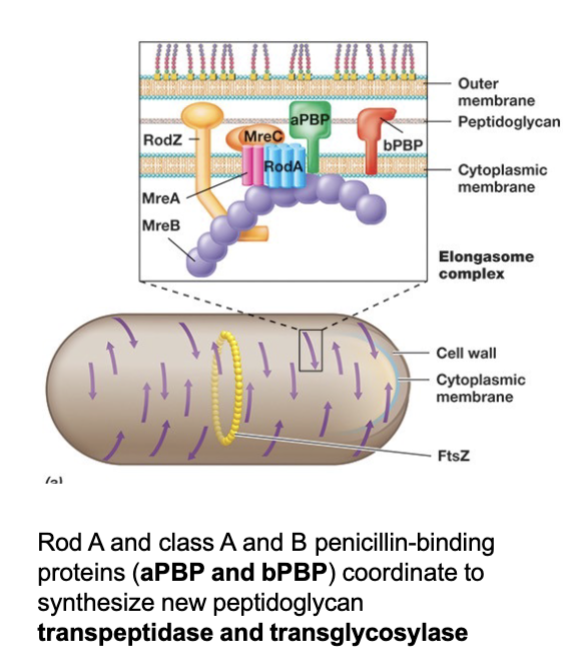
S-Layers of Archaea (Define and Function)
S-layers are common cell wall structures in Archaea and some Bacteria.
Composed of a protein or glycoprotein monolayer.
Functions:
Structural support (rigid layer).
Molecular sieve (controls permeability, semi-permeable).
Forms a pseudo-periplasmic space in some cells.
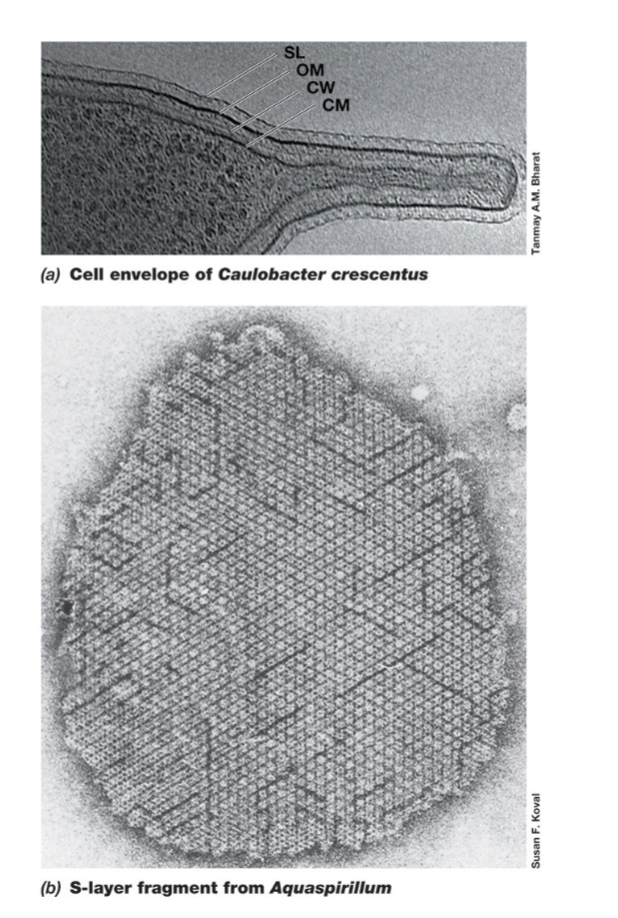
Capsule/exopolysaccharide/glycocalyx/slime layer (Function)
Capsule, exopolysaccharide (EPS), glycocalyx, and slime layer are external protective layers (terms are synonyms).
Functions:
Attachment to surfaces and biofilm formation.
Defense against grazing predators, immune responses, and antibiotics.
Prevents desiccation (drying out).
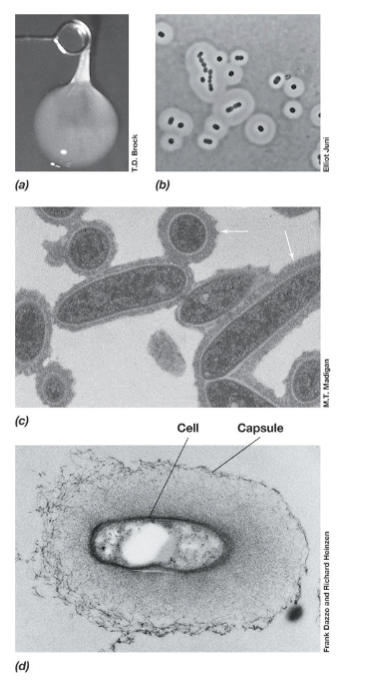
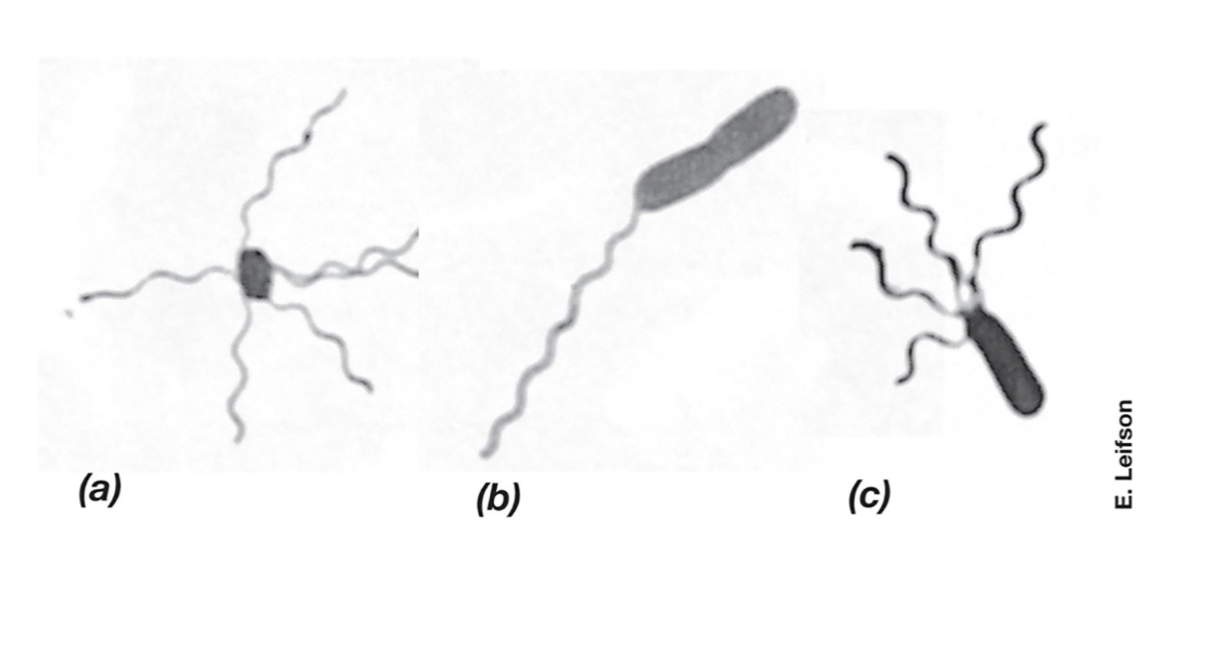
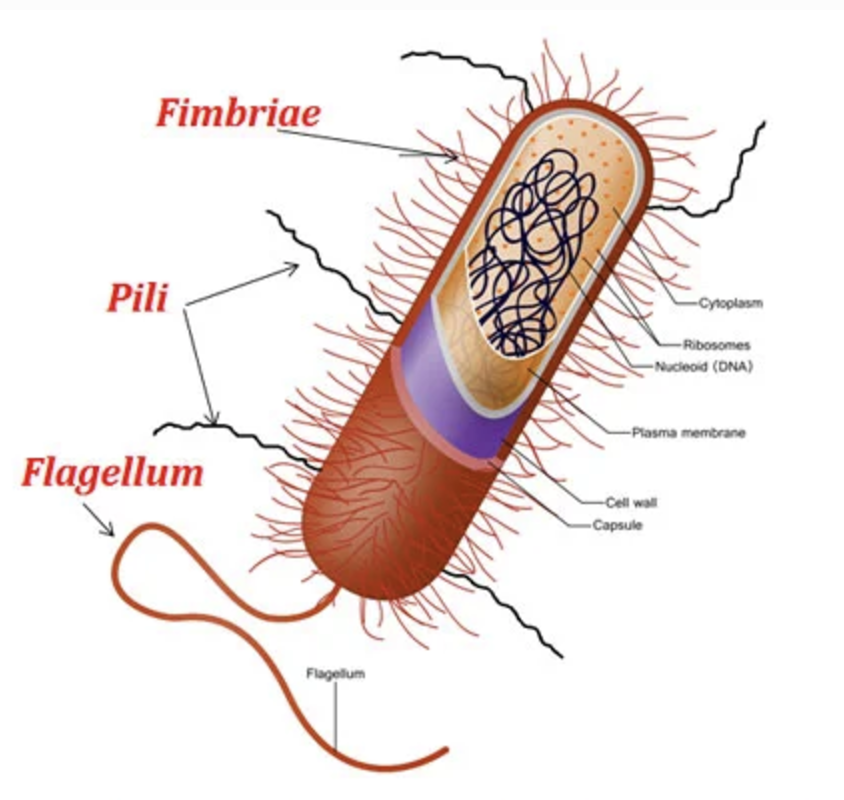
Flagella (Definiton and 3 types)
Flagella are long, whip-like structures used for motility in bacteria. They rotate like a propeller, allowing movement through liquid environments.
3 Types:
Monotrichous – A single flagellum at one end (e.g., Vibrio cholerae) (b).
Lophotrichous – A cluster of flagella at one or both ends (e.g. , Pseudomonas species) (c).
Peritrichous – Flagella spread all over the cell surface (e.g., Escherichia coli) (a).
Pili and Fimbriae (Definiton and Differences)
Fimbriae and pili are thin, filamentous protein structures (2–10 nm wide).
Fimbriae help bacteria stick to surfaces and form pellicles (thin biofilms on liquid surfaces).
Pili are longer, thicker, and fewer than fimbriae.
Conjugative pili enable genetic exchange between cells (conjugation).
Type IV pili assist in host adhesion and twitching motility (e.g., Pseudomonas, Moraxella).
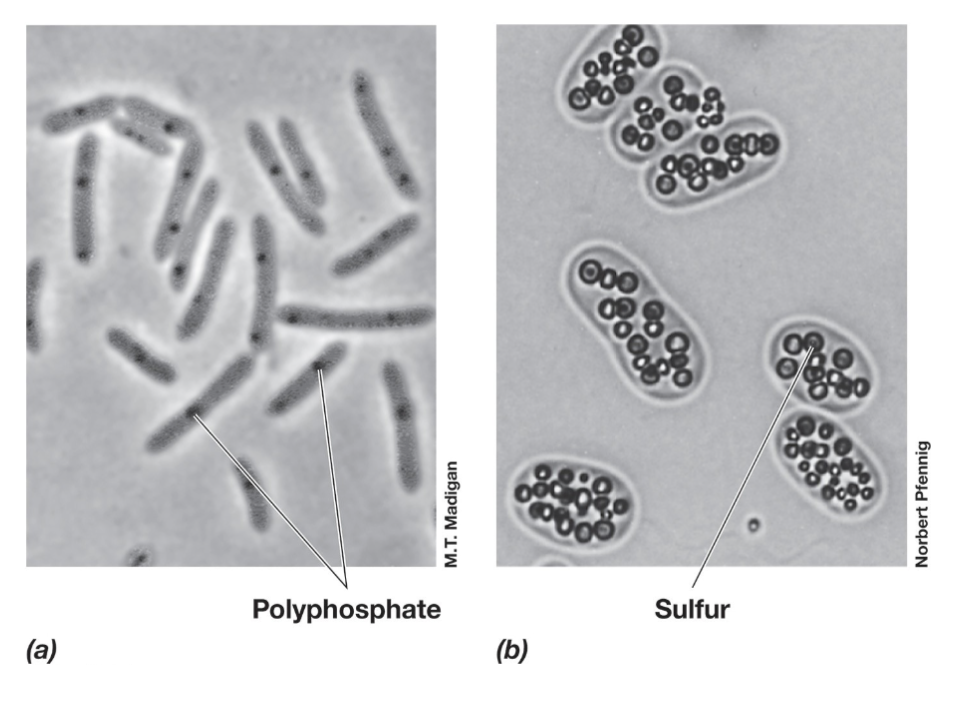
Inclusion Bodies
Inclusion bodies are compact aggregates of stored materials within bacterial cells.
Carbon & Energy Storage Polymers:
PHA (polyhydroxyalkanoates) – Lipid family, includes PHB (poly-β-hydroxybutyrate).
Glycogen – Stored as a carbon and energy reserve.
Polyphosphate Granules – Store inorganic phosphate for energy and biosynthesis.
Sulfur Globules – Store elemental sulfur in the periplasm, which can be oxidized to sulfate (SO₄²⁻).
Carbonate Minerals – Bacteria can biomineralize barium, strontium, and magnesium.
Protein Bound Compartments (and Functions) (2)
Chlorosomes: light-harvesting complexes found in green sulfur bacteria: allow bacteria to grow at low intensities.
Carboxysomes: protein-enclosed organelles found in cyanobacteria and some bacteria, where carbon fixation occurs by housing key Calvin cycle enzymes like RuBisCO for efficient CO₂ assimilation.
Gas Vesicles (Function)
Buoyancy regulators in some planktonic bacteria.
Magnetosomes (Function)
Magnetic iron oxides allow cell to undergo
magnetotaxis: migration along magnetic field lines.
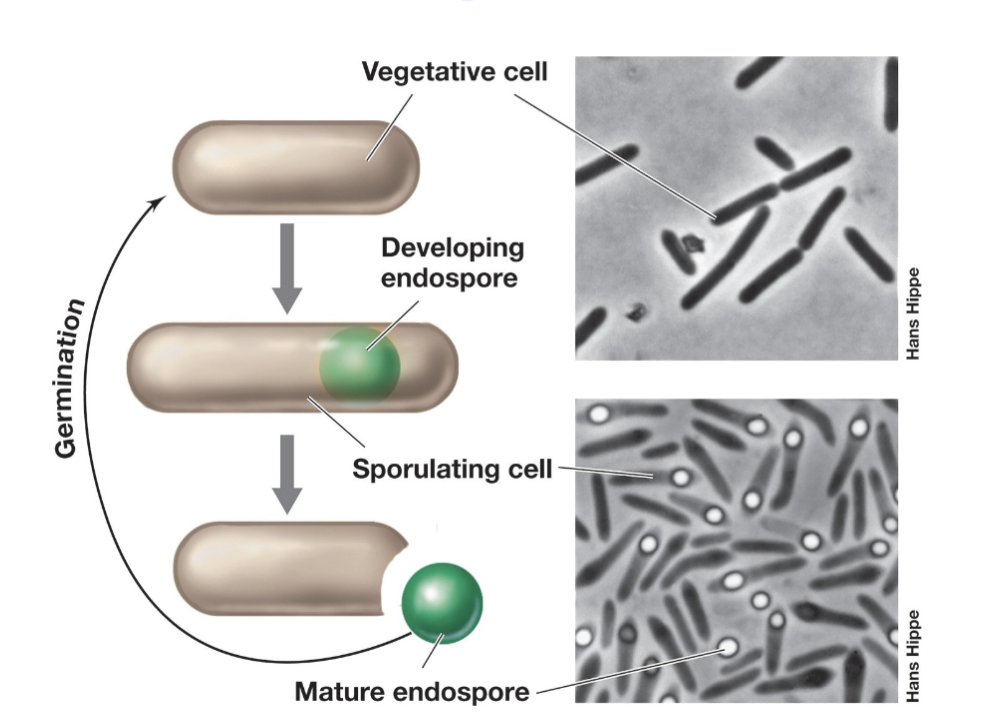
Endospores (Function)
Endospores are highly resistant to heat, chemicals, and radiation.
Serve as the dormant stage of the bacterial life cycle.
Ideal for dispersal via wind, water, or animal gut.
Found only in some Gram-positive bacteria (e.g., Bacillus, Clostridium).
Formed when growth stops due to lack of essential nutrients (e.g., carbon, nitrogen).
Can remain dormant for years but quickly rehydrate and return to vegetative form when conditions improve.
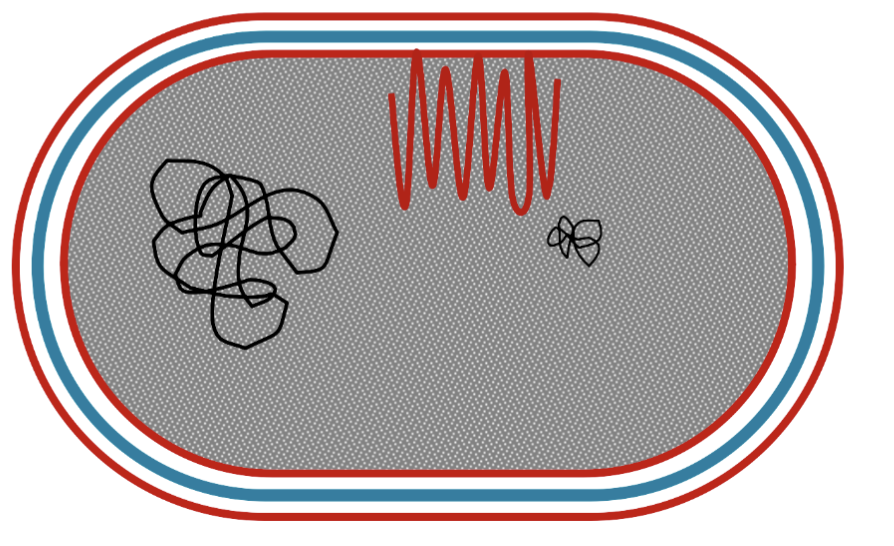
Internal Membrane Folding (Function)
Internal membranes in certain bacteria (e.g., phototrophic, nitrifying, methanotrophic) are extensions of the cytoplasmic membrane, not separate structures.
These membranes are invaginations (folds) of the cytoplasmic membrane.
Key enzymes for processes like photosynthesis, nitrification, or methane oxidation are bound to these membranes.
The more membrane surface area present, the more space for these enzymes, enhancing the bacterium's ability to carry out its specific metabolic processes.
Nitrifying Bacteria
Nitrification is the lithotrophic oxidation of ammonia (NH₃) to nitrate (NO₃⁻), typically occurring in two separate reactions by different bacteria.
Ammonia oxidizers (e.g., Nitrosococcus) oxidize NH₃ to NH₂OH.
Nitrite oxidizers (e.g., Nitrobacter) convert NO₂⁻ to NO₃⁻.
Many nitrifying bacteria have internal membrane systems that house the key enzymes for nitrification:
Ammonia monooxygenase: oxidizes NH₃ to NH₂OH.
Nitrite oxidase: oxidizes NO₂⁻ to NO₃
Special Functions of Planctomycetes
Some Planctomycetes have unique internal membrane structures:
Anammoxosome: membrane-bound compartment for anaerobic ammonia oxidation (anammox process).
Nucleoid: membrane-bound DNA compartment, unlike most bacteria.
These structures enable specialized functions and show eukaryote-like compartmentalization.
Habitat (Definition)
Parts of an ecosystem suited to a particular
group of populationsProkaryotes have broader ecosystem ranges than eukaryotes.
Habitat Composition (Just know categories)

Microbial communities can be described in terms of (2):
Species richness: the total number of different
species presentSpecies abundance: the population size of each
species in an ecosystem
Guilds (Definition)
Metabolically-related microbial populations
Perform key steps in biogeochemical cycles
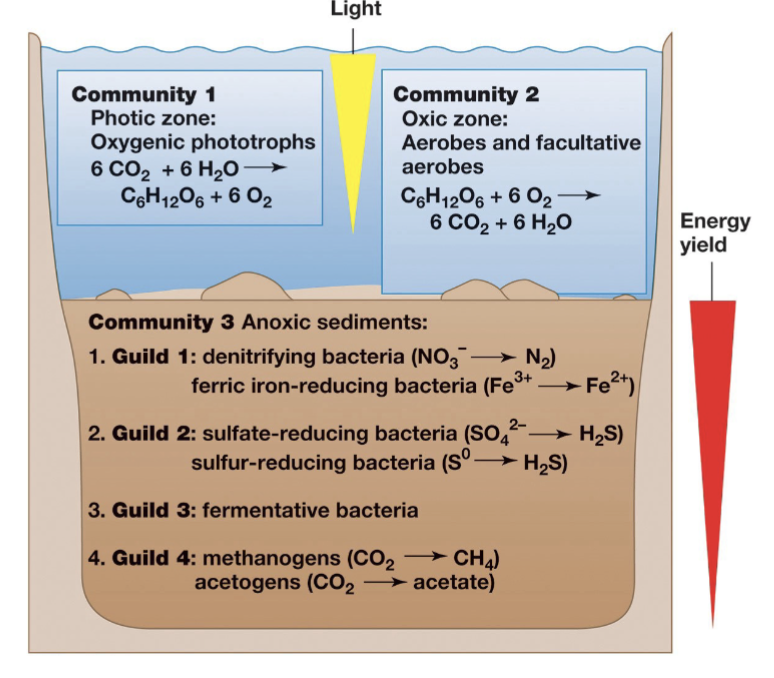
Environments and Microenvironments
A microenvironment is the small area where a species interacts with its surroundings.
Physicochemical conditions (e.g., temperature, pH) in microenvironments change rapidly in both space and time.
Resources in nature are highly variable, and microbes often experience feast-or-famine conditions.
Growth rates in natural environments are typically lower than those in controlled lab settings.
Competition and cooperation occur among microbes in natural environments.
Soil Composition and Function
Soil Composition:
Inorganic mineral matter (~40%)
Organic matter (~5%)
Air and water (~50%)
Living organisms
Limiting factors for microbial activity:
Water availability often limits activity in surface soils.
Energy sources (organic matter) and inorganic nutrients are also key limiting factors.
The rhizosphere:
The area around plant roots where plants secrete sugars and other compounds.
Rich in organic matter and microbial life.
There are diverse microenvironments in soils and
microbial diversity is very high
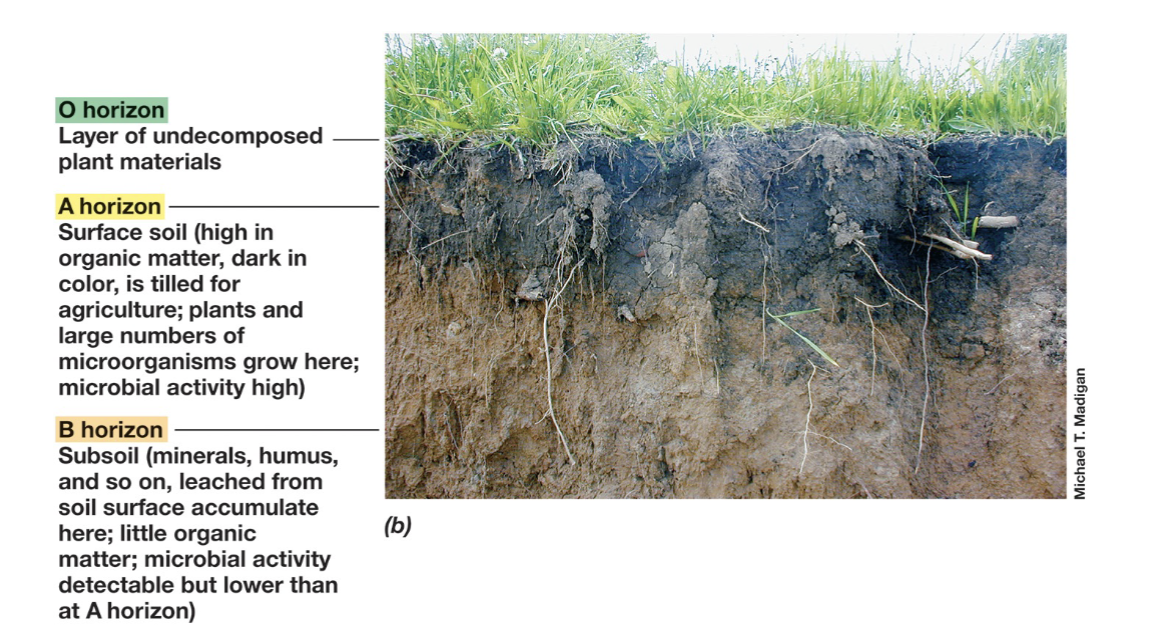
O,A, and B Horizon in Soils
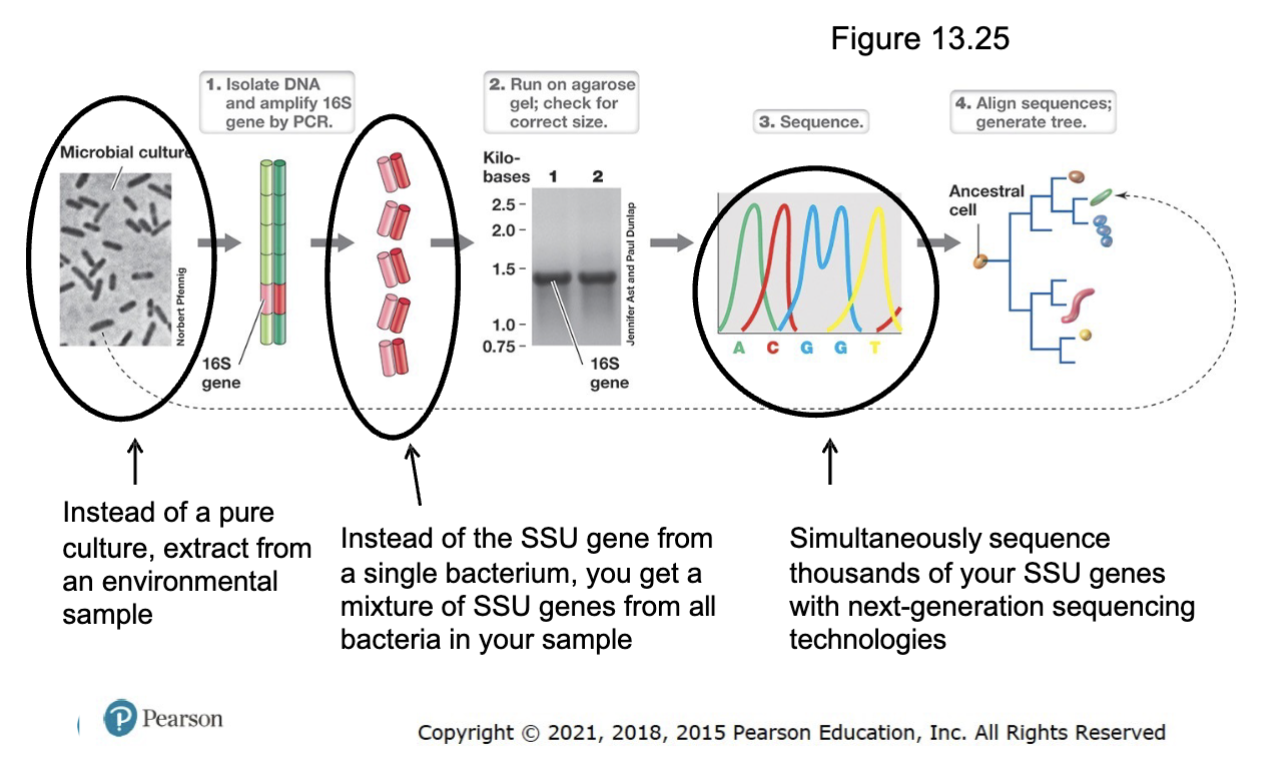
How to identify bacteria in an environmental sample based on SSU rRNA sequences
Extract DNA from the environmental sample (not pure culture).
Amplify the SSU rRNA gene (16S rRNA in bacteria) using PCR with universal primers.
Instead of the SSU gene froma single bacterium, you get a
mixture of SSU genes from all bacteria in your sample.
Sequence the amplified genes to obtain the SSU rRNA sequence.
Compare the sequence to known databases and identify bacterial species.
Analyze phylogenetic relationships to classify bacteria within broader taxonomic groups.
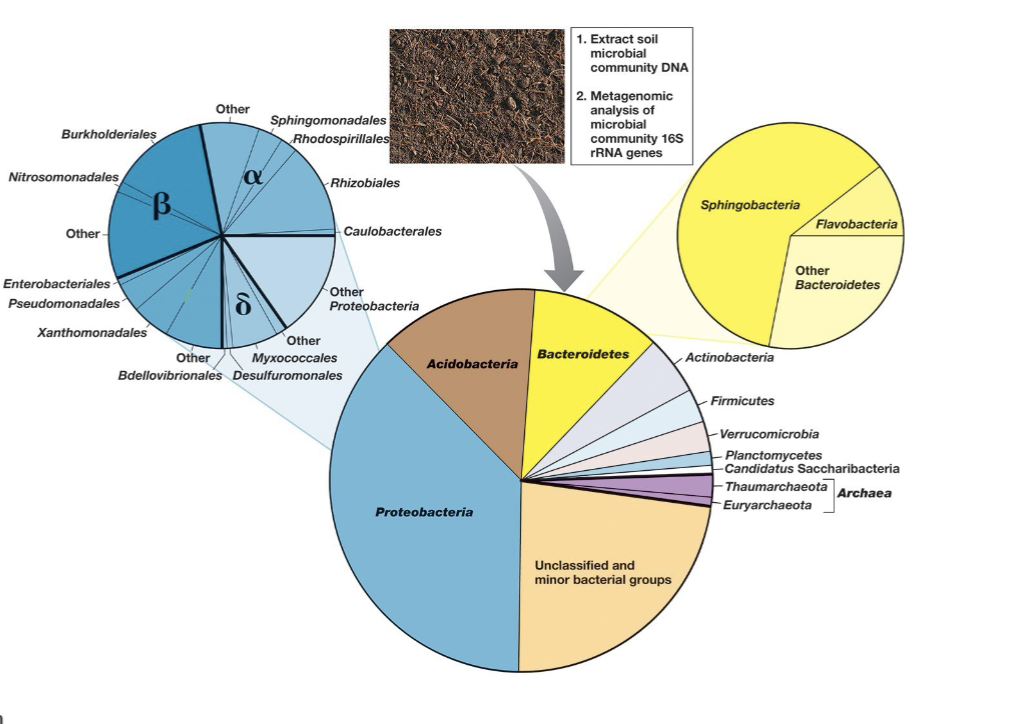
Soil Community Analysis and OTU
OTU (Operational Taxonomic Unit): A 16S rRNA gene sequence differing by >3% from others, typically representing a species.
Molecular sampling shows thousands to hundreds of thousands of microbial species (OTUs) in most soils.
Microbial diversity varies based on soil type and geographical location.
Nearly all 16S rRNA genes recovered from
environments like soil do NOT match cultured
species at >97% identity (or 98.7%)
• There are about 13,000 cultured, named species BUT
an estimated 1 million to 1 trillion uncultured species!
Soil Bacteria and Archaeal Diversity (General Composition)
Why are so many bacteria hard to cultivate?
Environmental conditions in labs differ significantly from natural habitats, making it hard to replicate the complex microenvironments where many bacteria thrive.
Many bacteria have specific nutrient or growth requirements that are not easily provided in standard laboratory media.
Growth rate limitations: Some bacteria grow extremely slowly or require cooperative interactions with other microbes that are hard to mimic in isolation.
Uncultivable species may rely on symbiotic relationships or specialized metabolic pathways that are difficult to recreate outside their natural setting.
Microbes in Coastal and Open Ocean Waters:
Oceans play a key role in Earth's carbon balance due to significant microbial activity.
Near-shore marine waters have higher microbial numbers than the open ocean, primarily due to higher nutrient levels.
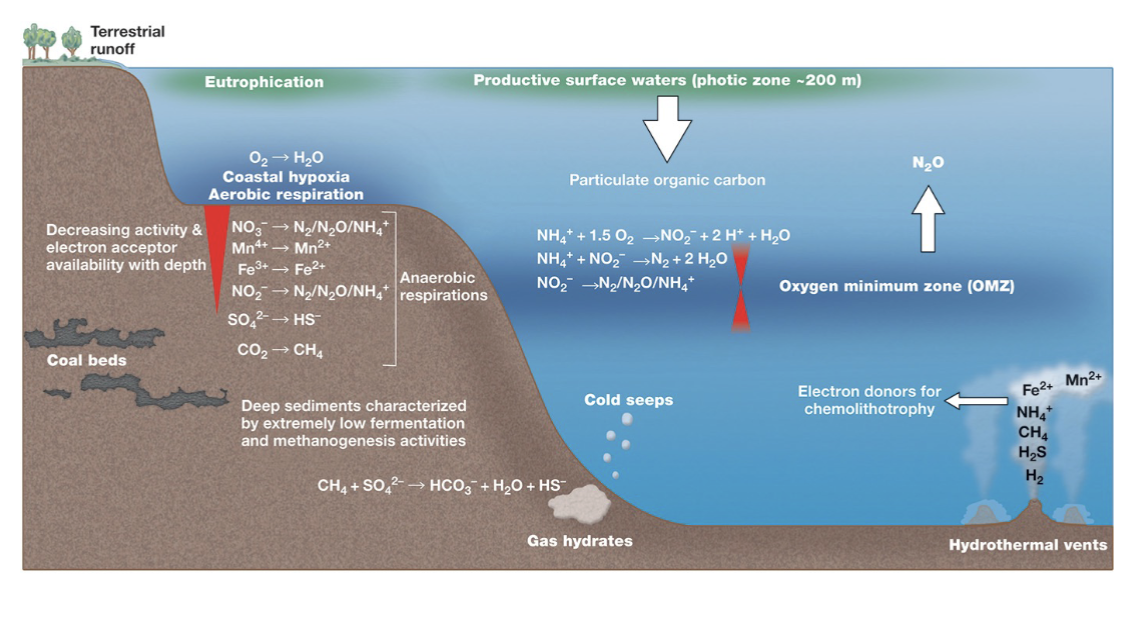
Coastal Ocean Bacterial and Archaeal Diversity (General Composition)
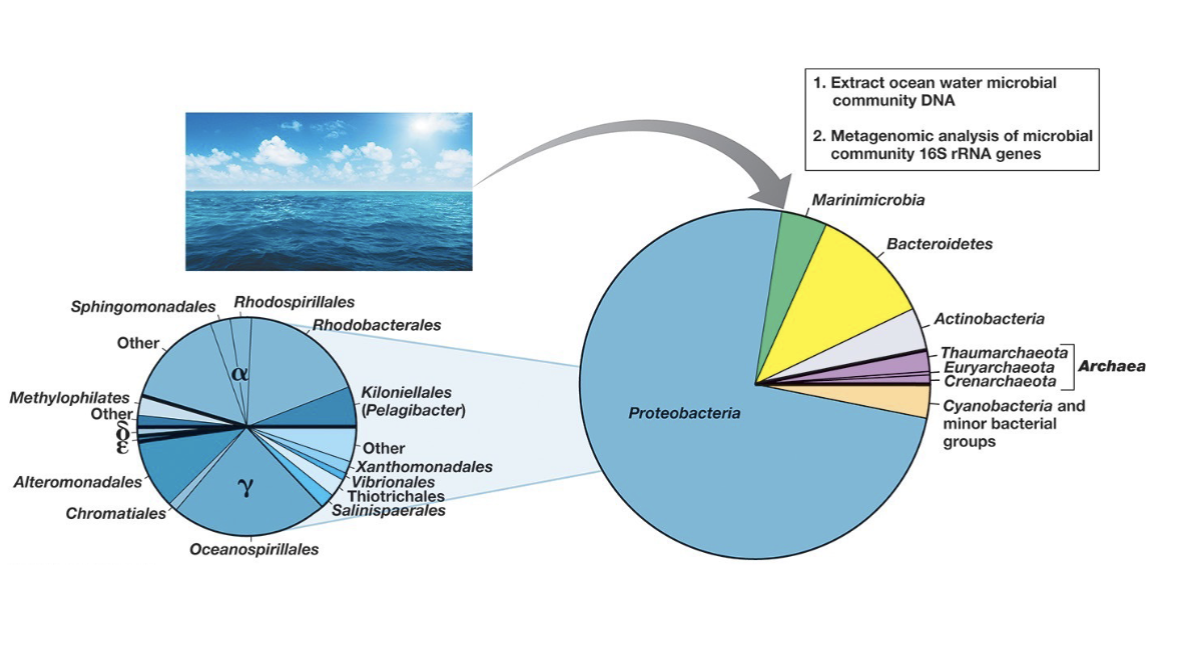
Coastal and Ocean Waters: Phototrophic Microorganisms
Primary productivity in the open ocean is mainly driven by photosynthesis from Cyanobacteria.
Prochlorococcus accounts for:
>40% of the biomass of marine phototrophs.
~50% of the net primary production in the ocean.
Pelagic (open ocean) Bacteria
The most abundant marine organoheterotroph is Pelagibacter, an oligotroph.
Oligotrophs thrive in very low nutrient concentrations.
Pelagibacter was known from molecular DNA studies as the most abundant ocean bacterium, but it took decades to grow a strain and is still not validated as a true species.
Pelagibacter contains proteorhodopsin, a form of rhodopsin that allows it to use light energy for ATP synthesis.
Marine Environment: Oil Spill Example
Deepwater Horizon oil spill
Largest marine oil spill ever
Oil released as a plume at great depths
Bloom of hydrocarbon-degrading Gammaproteobacteria, Colwellia, and Cycloclasticus
Early growth of hydrocarbon-degrading bacteria reduced the
environmental impact
Deap Sea Conditions
>75% of ocean water is deep sea, primarily between 1,000 and 6,000 meters deep.
Deep sea organisms must adapt to:
Low temperatures
High pressure
Low nutrient levels
Absence of light energy, leading to a prevalence of lithotrophs and extremophiles.
Planktonic vs. Attached Bacteria
Microbes can be planktonic (floating freely) or attached to a surface.
Biofilms are groups of bacterial cells that:
Adhere to a surface.
Are enclosed in an adhesive matrix secreted by the cells.
The matrix is typically made of polysaccharides.
Biofilms Formation
Biofilm formation begins when a cell attaches to a surface, triggering the expression of biofilm-specific genes.
These genes encode proteins that initiate matrix formation.
Quorum sensing (sensing population density) is essential for the development and maintenance of biofilms.
The primary quorum sensing molecules are acylated homoserine lactones
Biofilm Function
Bacteria form biofilms for several reasons:
Self-defense: Biofilms resist phagocytosis by immune cells and the penetration of toxins (e.g., antibiotics).
Nutrient trapping: Biofilms trap nutrients for growth and help prevent cell detachment in flowing systems.
Cell association: Biofilms allow bacteria to live in close association, facilitating cross-feeding and symbioses.
Biofilms in Human Settings:
Biofilms are linked to several medical and dental conditions, including:
Periodontal disease, cystic fibrosis, tuberculosis, Legionnaires' disease, and Staphylococcus infections.
A significant problem with medical implants like catheters and artificial joints.
In industrial settings, biofilms can:
Slow liquid flow through pipelines.
Accelerate corrosion, causing billions of dollars in damage (e.g., oil pipelines).
Very few effective antibiofilm agents are available.
Microbial Mats
Are very thick biofilms.
Built by phototrophic and/or chemolithotrophic bacteria
– Phototrophic mats have existed for over 3.5 billion years
(stromatolites)
– Often occur in systems with low predation/grazing, e.g.
extreme ecosystems
Extremophiles
Organisms that prefer conditions outside the limits of what is “normal”.
Environmental Stresses
Environmental factors affecting organism growth:
Temperature, pH, pressure, water activity/salt, oxygen concentration, radiation.
Organisms that grow in specific ranges of these factors are called -philes (e.g., thermophiles, acidophiles).
Organisms that can survive in these ranges but don't grow optimally are called -tolerant (e.g., halotolerant, acid-tolerant).
Cardinal Temperatures (3)
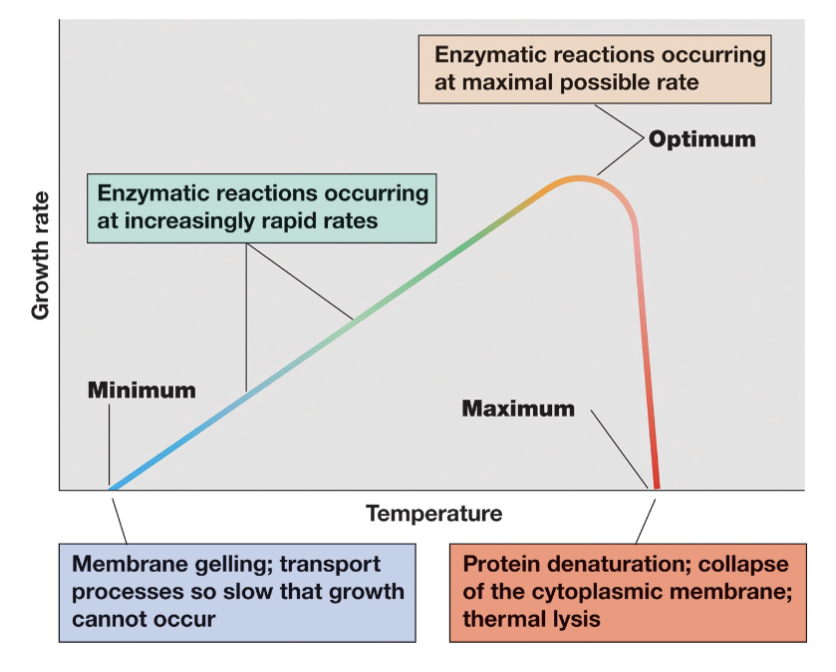
Thermophilic Habitats
compost, decaying organic matter
the deep biosphere (30 C increase for every 1 km depth)
geothermal systems, hot springs, mud pools undersea vents, etc.
Hyperthermophiles
Can grow above 80°C
Archeae are the best,
There are also some hyperthermophilic bacteria but no eukarya can grow above 62°C.
Different processes have different maxima (eg. photosynthesis stops at 73°C)
Microbes around Hydrothermal Vents
Thermophiles and hyperthermophiles thrive in high-temperature environments.
Chemolithotrophic prokaryotes at deep-sea hydrothermal vents utilize inorganic materials (e.g., S, H2S, H2, Fe2+, Mn2+) for energy.
Animal and microbial communities flourish around these deep-sea hydrothermal vents.
In these communities, bacteria are the primary producers, any animals are eating the microbes or have symbiotic microbes.
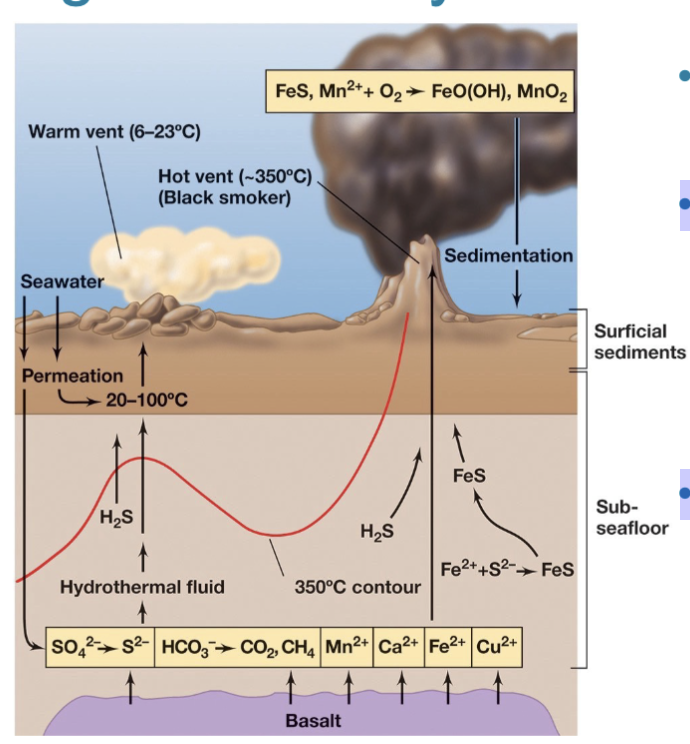
Microbial Life at High Temperatures Problems (3)
High temperature increases reaction and growth rates, but only up to a point.
Negative effects of high temperatures on cells:
Proteins denature.
DNA/RNA denature.
Membranes become too fluid.
Microbial Life at High Temperatures Solutions (3)
1) Stronger bonds to stabilize proteins
2) Increase DNA/RNA stability (GC content, reverse gyrase)
3) Decrease membrane fluidity (tetra-ethers in archeae)
Microbial Life at High Temperatures: Proteins
Thermophilic enzymes function optimally at high temperatures due to several features that provide stability:
Critical amino acid substitutions create heat-tolerant protein folds.
Increased ionic bonds between basic and acidic amino acids resist unfolding.
Hydrophobic interiors help maintain protein structure.
Solute production (e.g., di-inositol phosphate, diglycerol phosphate) stabilizes proteins.
Smaller, spherical proteins with less quaternary structure are more stable.
Microbial Life at High Temperatures: RNA/DNA
DNA/RNA stability at high temperatures is increased via:
Positive supercoiling of DNA via reverse gyrase: stabilizes the DNA structure, preventing denaturation and maintaining its integrity under thermal stress.
RNA stability increases with higher G-C content (have 3 H-bonds compared to 2 of A-T/U)
(mRNA is linear, but secondary folding structure is critical to tRNA and rRNA function).