Allotropes of carbons
1/12
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
What are the properties of diamond?
Really hard: because of the 4 covalent bonds per carbon atom
High melting point: requires a lot of energy to break the covalent bonds
Doesn’t conduct electricity: no delocalised electrons
What and why are the properties of graphite?
Soft and slippery → lubricating material: weak bonds between the hexagon layers
High melting point: covalent bonds in the layers require a lot of energy to break
Conducts electricity and heat: each carbon atom has one delocalised electron
What is graphene?
A sheet of carbon atoms joined together in hexagons. Its 2 dimensional and only 1 atom thick
What are the properties of graphene?
Strong: covalent bonds
Light
Conducts electricity: has delocalised electrons like graphite
What is graphene used for?
to strengthen composite materials
Used in electronics
What are fullerenes?
molecules of carbon shaped like closed tubes or hollow balls
What are fullerenes made of?
Carbon atoms arranged in hexagons, pentagons or heptagons
What are fullerenes used for?
Cage: can be used to deliver drugs around the body
Catalysts: huge surface area
Lubricants
What is buckminsterfullerene?
The first fullerene to be discovered
C60 and forms a hollow sphere
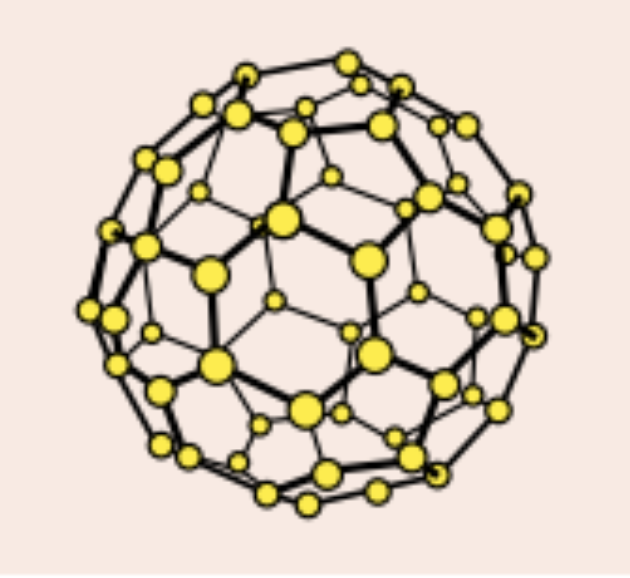
What are nanotubes?
Tiny carbon cylinders
What are the properties of nanotubes?
Length:diameter ratio is high
Conducts electricity and heat
High tensile strength( don’t break when stretched )
What is nanotechnology?
Technology using very small particles such as nanotubes
What are nanotubes/nanotechnology used for?
Electronics
strengthen materials without adding weight