principles of infectious disease - bacterial toxins
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
56 Terms
non protein toxins
Endotoxin; embedded in the cell surface: Lipopolysaccharide (LPS)
Other bacterial cell wall components; e.g. Lipoteichoic acid (LTA) & phosphatidylglycerol (PG)
Mycolactone; polyketide-derived macrolide
produced & secreted by some mycobacteria
Both cytotoxic and immunosuppressive properties
protein toxins (exotoxins)
excreted from the cell
type one (superantigens)
type II (phospholipases, pore forming toxins)
type III (A-B) toxins
features of endotoxins
formed from lipopolysaccharides that are part of the outer membrane
they cannot be denatured by boiling
relatively low potency and dont have enzymatic activity
pyrogenicity = fever causing
can cause septic shock - an inflammatory response throughout the body
features of exotoxins
are proteins
extracellular and diffusible
usually denatured by boiling
are antigenic and when denatured form a toxoid with antigenic properties but no longer toxic
relatively high potency and usually act as enzymes
only occasionally pyrogenicity
inflammatory response
4 major events of the inflamatory response
Vasodilation
Activation of endothelial cells
Increased vascular permeability
Chemotactic factors
Brought about by a combination of complement activation, cytokine release and monocyte and PMN transmigration/activation
roles of cytokines and complement in the inflammatory response
TNF alpha il-1 and IL-8 - all act on the brain causing - fever, anorexia, chills and wasting
also activate and attract phagocytes and mast cells
complement C3a C4a and C5a all activate and attract phagocytes and mast cells
IL-6 - stiumulates liver to release acute phase proteins = complement proteins (opsonising) and transferrin = drop in Fe in serum
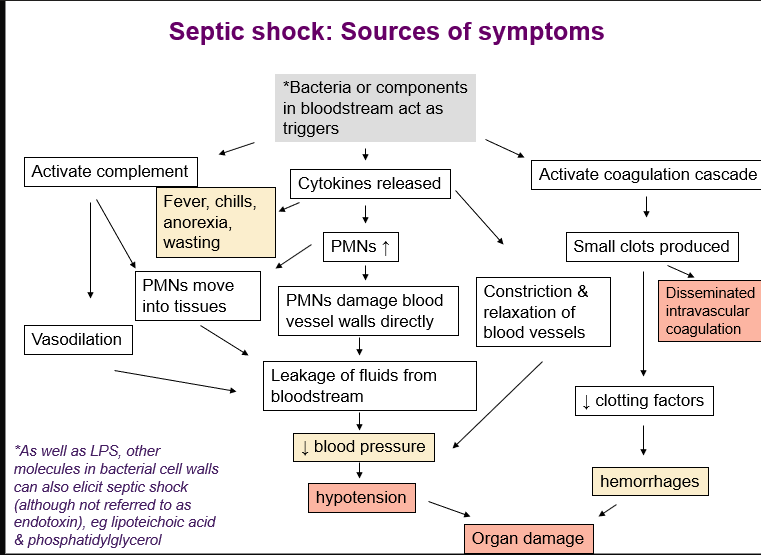
septic shock
Occurs in 4 stages
Systemic inflammatory response syndrome (SIRS)
Temperature > 38oC or <36oC (usually ↑)
Higher than normal respiratory rate
Usually high neutrophil count (sometimes low)
Mild hypotension
Sepsis
SIRS with proof bacteria in bloodstream
Severe sepsis
Multiple organ dysfunction
Dramatic hypotension
Septic shock
Hypotension despite fluid administration
disseminated intravascular coagulation
acute respiratory distress
multiple organ failure & death 50% survival + survivors can have amputation and organ damage
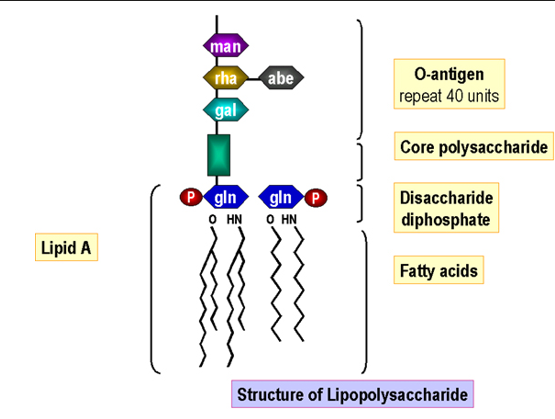
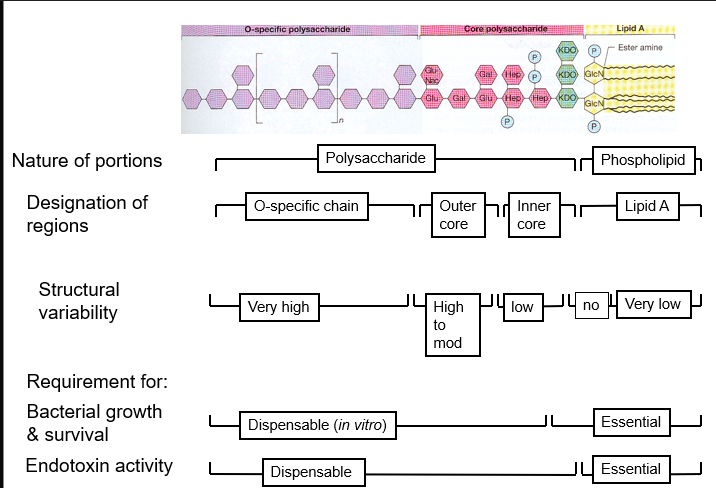
bacterial endotoxin structure
there is a relationship between their structure, function and biological activity
Lipid A part responsible for septic shock
Triggering of cytokine release by gram-ve LPS and the role in septic shock
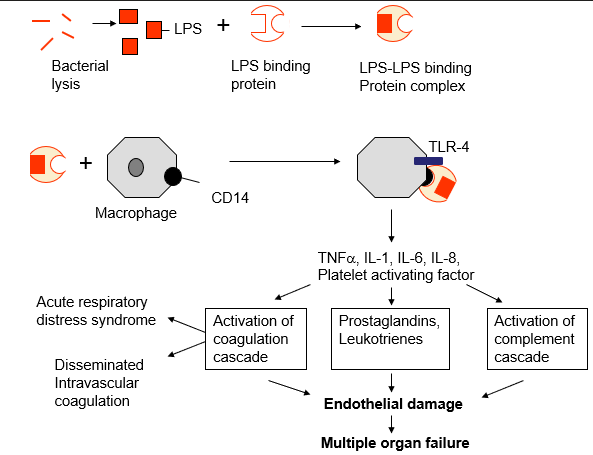
source of septic shock symptoms
bacterial exotoxin names
Cytotoxins: Attack a variety of cell types
Toxins that attack specific cell types include; neurotoxin, leukotoxin, hepatotoxin, cardiotoxin
Cholera toxin: produced by Vibrio cholerae
Shiga toxin: produced by Shigella species (dysentery)
Diphtheria toxin: produced by Corynebacterium diphtheriae
Tetanus toxin: produced by Clostridium tetani
Adenylate cyclase: toxin produced by Bordatella pertussis cause of whooping cough
Phospholipase (lecithenase): toxin produced by Clostridium perfringens cause of gangrene
Enterotoxin: protein toxins that cause diarrhoea or vomiting
toxic shock - identical to septic shock, but you don’t need bacteria in the bloodstream due to the extracellular nature of exotoxins
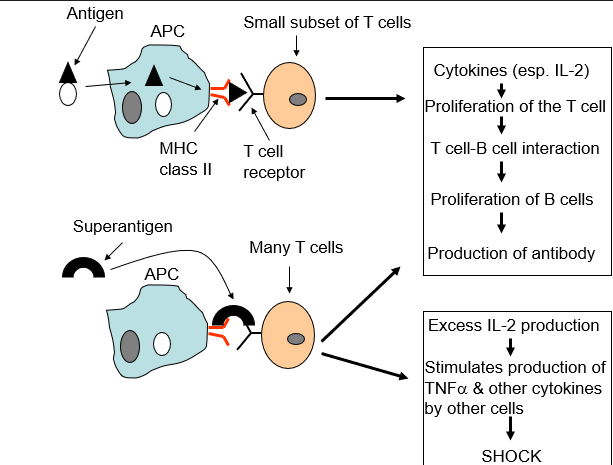
type I toxin
(superantigens)
Bind to the host cell surface, but are not translocated into the host cell
Antigen-independent activation of T cells
Inappropriate activation of IL-2 → TOXIC SHOCK
Exotoxins that act on helper T cells
Causes a massive release of nonspecific cytokines
Induce a systemic inflammatory response
type I toxin examples
Toxic shock syndrome toxin-1 (TSST-1)
Superantigen produced by some strains of Staphylococcus aureus.
causes toxic shock syndrome (TSS) - caused by a certain tampon brand
Staphyloccal enterotoxins (SEs)
Superantigens produced by some strains of Staphylococcus aureus
Cause food poisoning
Streptococcal pyrogenic exotoxin (Spe)
produced by group A streptococci (eg Streptococcus pyogenes).
causes toxic shock-like syndrome (TSLS)
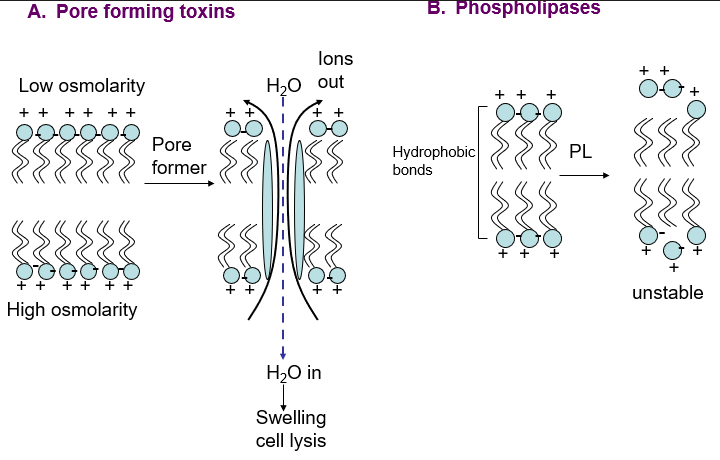
type II toxins
(pore forming, phospholipases)
Destroy the integrity of the mammalian cell cytoplasmic membrane
type II toxin examples
pore forming toxins
Staphylococcal a toxin
Produced by Staphylococcus aureus
Pore forming cytolysin
Listeriolysin O (LLO)
Produced by Listeria monocytogenes
Pore forming membrane damaging toxin, role in mediating escape from phagosome
Pneumolysin (PLY)
Produced by Streptococcus pneumoniae
Pore forming cytolysin
Induction of inflammation in lung
Inhibits cilial beat in respiratory mucosa
phospholipases
Clostridium perfringens a Toxin
Very common in the environment
Causes gas gangrene
Dermonecrotic & haemolytic
Is a zinc-metallophospholipase C
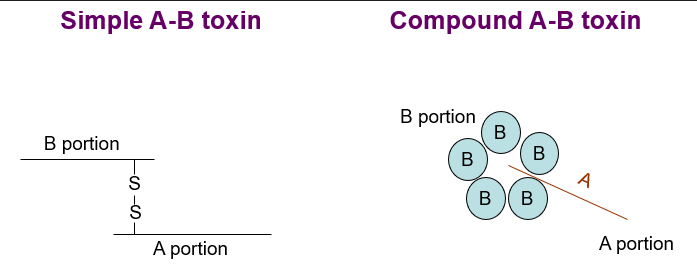
type III toxins
(A-B toxins)
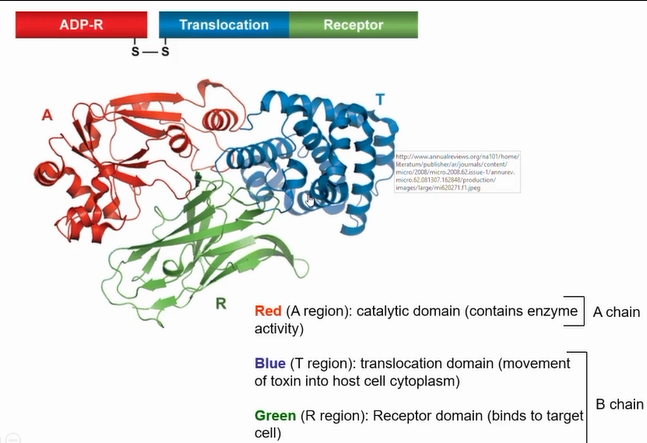
Have a B portion comprising a translocation (T) domain and a receptor binding (R) domain
Have an enzymatic portion (A) that acts on some intracellular host protein
Binding portion (B) and enzymatic portion (A) connected by disulphide bond
B portion confers specificity
A portion elicits effect on target host protein
Simple or compound
Number of outcomes dependent on target protein
what bacteria use type III AB toxins
Diphtheria
Botulism
Tetinus
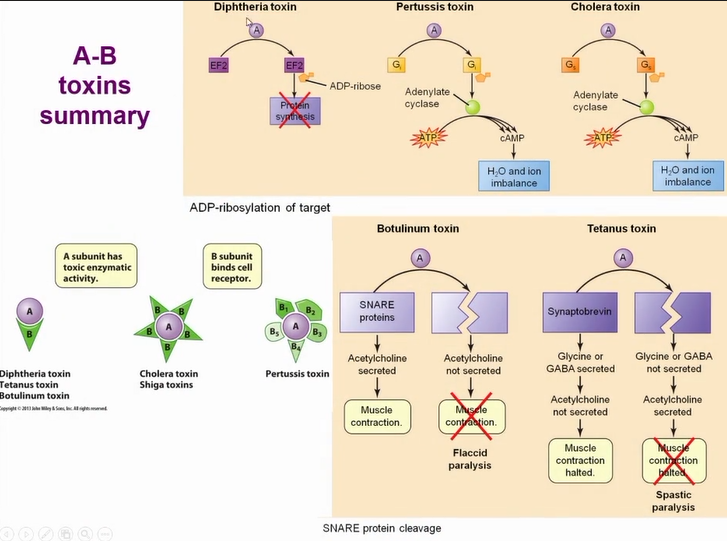
AB toxin structure
portions are held together by a disulfide bond the toxin only becomes active when the bond breaks
toxins of tetanus, botulism and diptheria are all simple AB toxins
A is the enzymatic part - targets protein in host cell
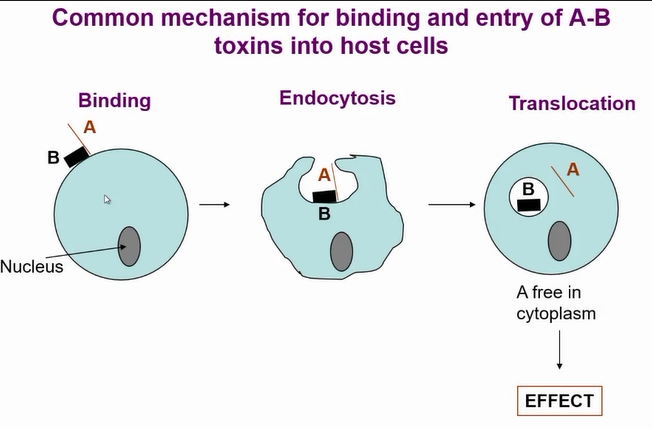
mechanism of AB toxin entry to cells
Common mechanism of entry for the toxins
B section mediates entry to cell
Once toxin is in the endosome the PH drop causes a change in the toxin and A portion travels into the cell and A portion is now active
there is a huge diversity of AB toxins due to receptors they bind to = very specific
most common action of AB toxins
A portion remove the ADP-ribosyl group from nicotinamide adenine dinucleotide (NAD and causes ADP-ribosylation of target host protein
resulting in inhibition of protein translation
diphtheria
Caused by Corynebacterium diphtheriae
Gram +ve, non-spore forming, facultative anaerobic rod
Humans are only host (normally childhood)
Transmission usually by inhalation of aerosols
Vaccine is a toxoided (chemically inactivated) version of diphtheria toxin
First symptoms (2-4 days post colonisation): malaise, low grade fever, tonsilitis, sore throat, loss of appetite
typically form a grey white membrane on tonsils and soft palete
complications
If complications: irregular heart beat, difficulty swallowing, stupor, coma & death
Bacteria produsces local toxin but it can go systemic
Toxin encoded onto bacteriophage in the genome
Only strains infected by the bacteriophage produce the toxin
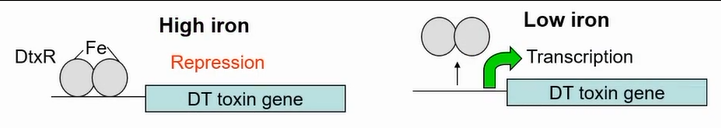
Toxing gene only made in low iron conditions (similar conditions to mucus in host)
High iron represses transcription allwoing it only to be expressed in the host
structure of diptheria toxin
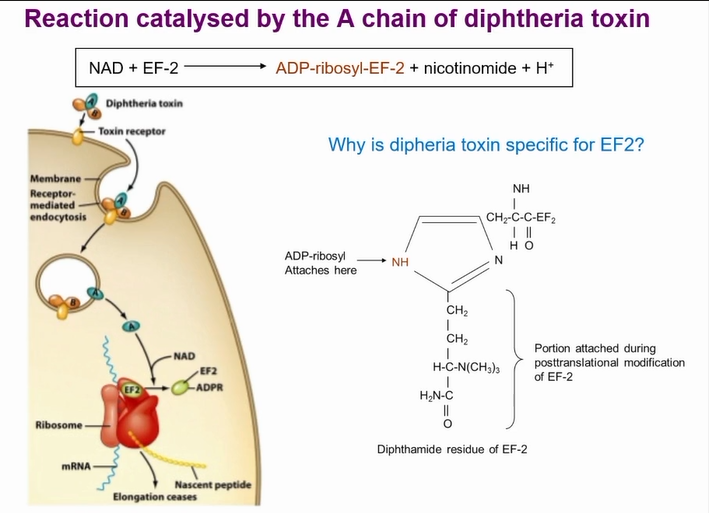
function of diptheria toxin
Toxin can get cleaved by protease on outside of the cell or cleaved by furine protease in endosome
Gets cleaved and A portion stays held to B portion by disulphide bond
B recognises receptor on host cell (HB-EGF receptor)
Now translocation domain exposed and due to hydrophobic nature intergrates into cell wall
Puts the A portion in the cell which breaks the disulphide bond
Toxin in cytoplasm
Stops protein synthesis by modifying EF-2
Is an enzyme so modifies EF-2 until it is all used up and leads to cell death
therapeutic uses for diptheria toxins
Therapeutic use of diptheria toxin - targeted cancer therapy
Diphtheria toxin derived immunotoxins
Use targeting molecules (receptor specific ligands) conjugated to modified toxin - targets toxin to cancer cells
Cancer cells frequently have specific growth factor receptors and antigens overexpressed on surface which allows selective targeting of immunotoxins
Targeting molecules are typically antibodies, antibody fragments, cytokines or growth factors that recognise a specific cell surface receptor that is either absent on the surface of normal cells or highly up-regulated on cancer cells
strategies based on controlled tumour specific expression of diptheria toxin
Strategies based on the controlled tumour-specific expression of diphtheria toxin
targeted killing of cancer cells by delivering botulinum A chain to cells and limiting its expression to within cancer cells through transcriptional regulation
If you can replace the receptor domain with the targetting molecule to lead the toxin to cancer cells
Issue is most of us are immune to the toxins and react to them
This is changes by introducing the enzyme portion of the toxin (A portion) via genetic engineering and get it to only be expressed in the cancer cells
botulism
Most cases caused by consumption of the toxin not colonisation by the bacteria
Intoxication caused by Clostridium botulinum
relatively large, anaerobic, Gram +ve, rods
forms sub-terminal endospores, strictly fermentative metabolism
Spores often contaminate food
Disease occurs when spores able to germinate and bacteria grow in food
Most cases associated with consumption of unheated canned food
Food heated but spores not destroyed and the bacteria replicates in anearobic environment
Disease symptoms proportional to amount ingested
botulism toxin
Botulinum toxins: potent neurotoxins - picograms can be deadly
Initial symptoms (4-36 hours after ingestion): nausea & vomiting, headache, double vision, slurred speech & other neurological symptoms
Generalised flaccid paralysis occurs due to blocking neurotransmitter release preventing muscle stimulation
Death if sufficient to interfere with breathing & heart function
Named after the latin for sausage as report comes from a fair in germany where people got ill from the sausage
Bacteria survives in farm animals intestine and spores get into fertiliser where food grows
three types of botulism
Other types of botulism are very rare
Most cases botulism outcompeted by microbiota but not in babies
Hence why you don’t feed honey to babies under one
Wound botulism only occurs in anearobic wounds (seen in war time)
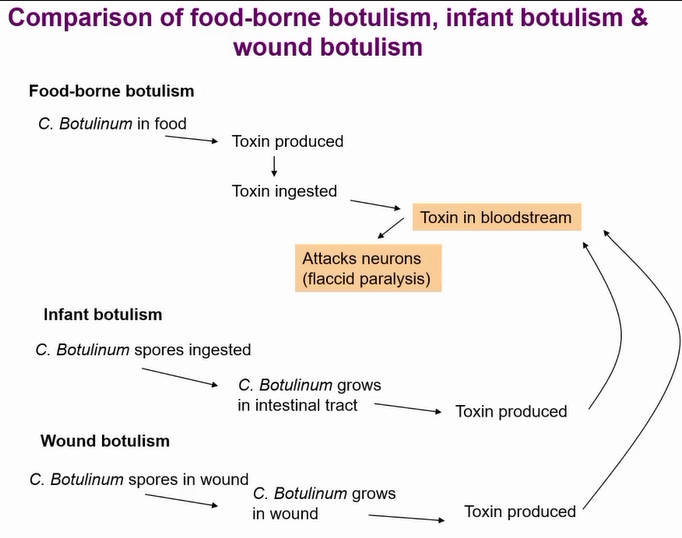
structure of botulism toxin
Multiprotein complex called progenitor toxin other proteins protect the toxin from low PH in stomach
If derivative toxin ingested its much less active as its not protected
If injected the derivative toxin is more deadly
Then its cleaved by protease and end up with A (light chain) and B chain (heavy chain)
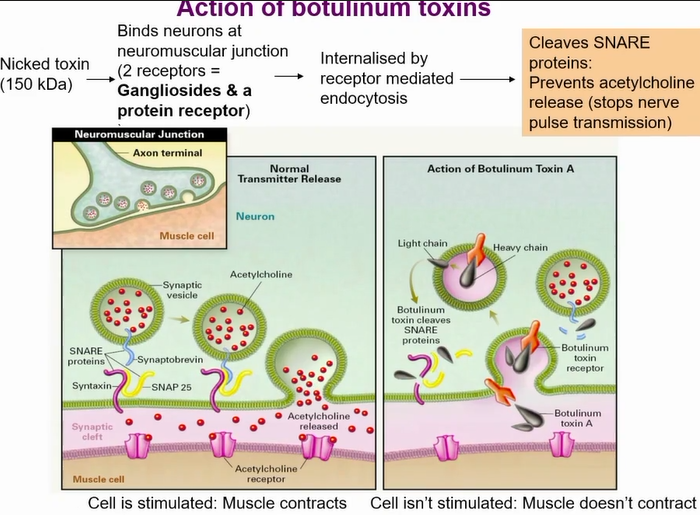
how the toxin gets in cells
Action of botulinum toxins
Uses 2 receptors to get in
Ganglioside to make lose connection the strong connection by a protein receptor
botulism toxin actions
Different serotypes cleaves different snare proteins
Block acetyl choline vesicle form fusing with cell membrane - no neurotransmission
results in flaccid paralysis
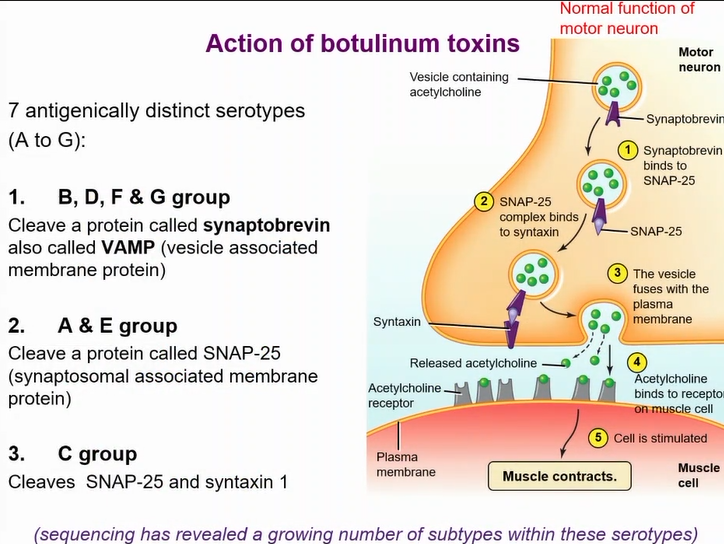
actions of diferent botulism serotypes
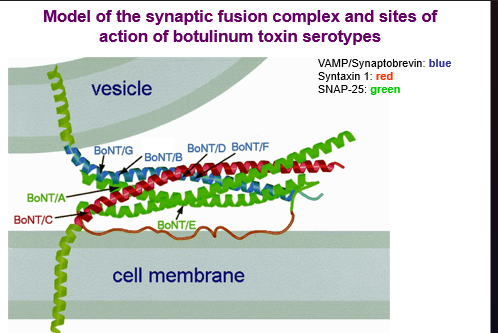
Tetanus
Intoxication caused by Clostridium tetani
relatively large, anaerobic, Gram +ve, rods
Forms terminal endospores, strictly fermentative metabolism
Spores found in soil
Disease occurs when spores germinate in wounds
Has to be a tight wound so its anaerobic
Vaccine is toxoided version of tetanus toxin
Tetanus toxin: potent neurotoxin
Highly fatal (40-80% mortality)
Early symptoms (4 days to several weeks): painful spasms and rigidity of the voluntary muscles (‘lockjaw’: spasms of the masseter muscle) - lock jaw
Progressive rigidity and violent spasms
Death usually from exhaustion & respiratory failure & occlusion of carotid in neck
action of tetanus toxin
Very similar to botulinam toxin and cleaves snare proteins
Targets inhibitory neurotransmitters
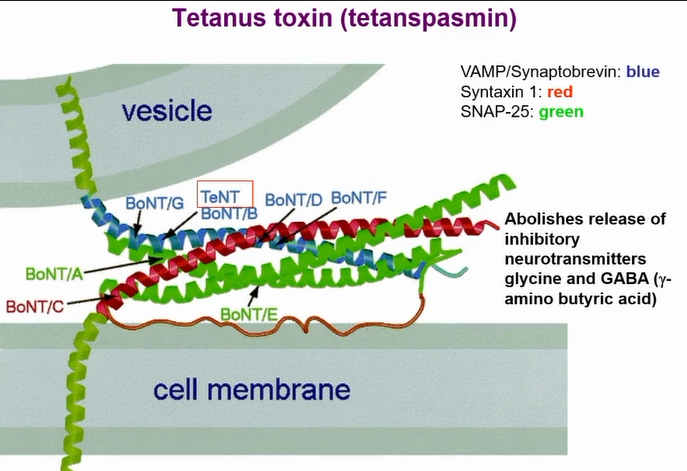
key differences between tetanus toxin and botulism
When botulism toxin goes in there is a PH drop in the endosome and the A chain comes out of it
For tetanus it associated with lipid rafts - no PH drop
Toxin goes through retrograde trafficking and goes into inhibitory neurons by trancytosis
Now PH drops in endosome and A chain comes out
In inhibitory neuron there is gamma and glycine
Does the same thing as botulism
therapeutic uses of botulism toxins
Native toxin administered into peripheral tissue resulting in reversible blockage of the neuromuscular junction:
Dystonias and other involuntary movements, eg:
Face and neck dystonias
Limb, head, voice, chin tremor
Inappropriate muscle contractions, eg:
Spasticity
Chronic tension (muscle contraction) headaches
Neuropathic pain
Neurogenic bladder
Hyperhidrosis - excessive sweating
Sialorrhea - excess saliva within the oral cavity
May result from stroke, cerebral palsy, head injury, multiple sclerosis etc
Other cosmetic applications include:
wrinkles, brow furrows, frown lines, "crow’s feet", platysma lines, facial asymmetry
Commercial preparations include:
From BoNT/A: Dysport®, Botox®, Xeomin® & CBTXA®
From BoNT/B: Myobloc® (also known as NeuroBloc ®)
cholera
Caused by water/ food contaminated with human feaces
Causative agent: Vibrio cholerae
Gram –ve, curved rod, motile (single polar flagelllum)
capable of respiratory & fermentative metabolism
Transmission to humans is by water or food
Reservoirs: Infected humans esp. asymptomatic carriers (marine invertebrates)
Can survive in water but need to be in human to replicate in intestines
Disease due to secretion of enterotoxin: cholera toxin
Highly fatal (50-60% mortality) and very rapidly fatal death within hours potentially
Symptoms (18h to 5 days): sudden onset of diarrhoea (‘rice water’ stool)
Leads to dehydration, anuria (kidneys stop urine production), acidosis(low PH in blood) & shock
Loss of potassium ions leads to cardiac complications & circulatory failure→death
Prevention: clean water supply, effective sewage treatment
Treatment: Fluid replacement therapy
Vaccine recently became available
identification of cholera
start largely from indian sub continent
Identified by koch (kochs postulates)
Became more virulent with the el tor strain which took over in 7th pandemic
In 8th pandemic it went through mutation in the el tor strain and could infect people who were immune to previous strains
Still kills millions and is still endemic in many areas
Every so often you end up with a pandemic
virulence factors of cholera
Motility
Flagellum
Acf (accessory colonisation factor) gene products regulate motility
Neuraminidase
penetration of mucin layer
Adherence
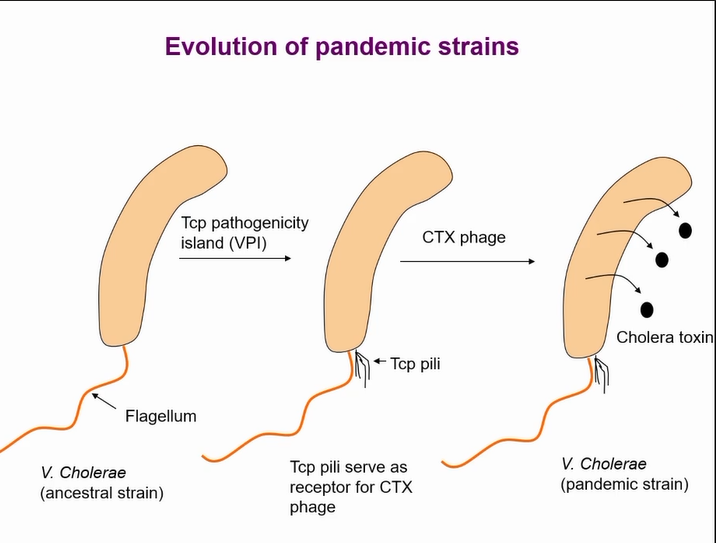
Toxin coregulated pili (Tcp pili)
clumped at one pole of the cell
Tcp genes (except tcpG) are clustered on a pathogenicity island (VPI)
Non-fimbrial adhesins: Haemagglutinins
The genes responcible for the TCP pilli clustered on a gene island that has other adhesion but most important adhesion is the TCP pili
Cholera toxin
Other toxins produced by some strains
Zot (zonula occludens toxin)
Ace (accessory cholera enterotoxin)
ChxA: not in O1 & V. cholerae O139 strains
Other toxins can cause symptoms in absence of the cholerae toxin but it is still the main toxin
acquisition of virulence
Two key events allowing the bacteria to cause toxin that results in pandemics
Once the pathogenicity island thought to be acquired by a phage but that is now controversal
Pilli acts as a receptor for the phage to intergrate the toxin
pathogenesis of cholera
Pathogenesis of cholera
V. Cholerae ingested
Virulence genes expressed
(pH shock in stomach, t temp)
Adheres to & colonises small-intestinal mucosa (flagella, TCP pili, other adhesins?, neuraminidasg?)
Produce toxins (cholera toxin, Zot?, Ace?)
Cholera toxin acts on mucosal cells
Extensive fluid & ion loss from tissues leading to hypotension, electrolyte imbalance & death
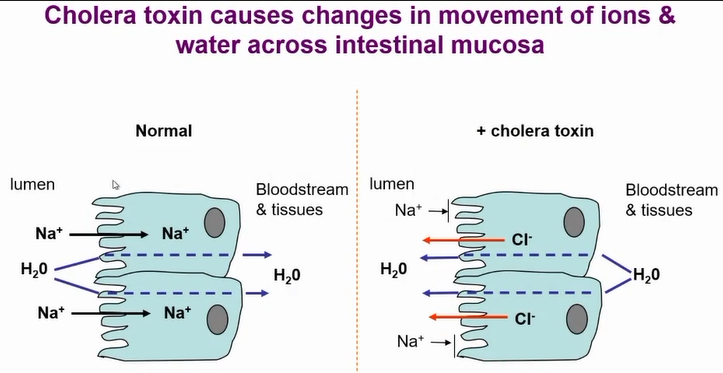
action of cholera toxin
resulrs in changes in the movement of ions and water across intestinal mucosa - cl- out and NA+ blocked from entering
results in massive loss of water from cells
gene structure of cholera toxin
Symptoms caused by cholera toxin
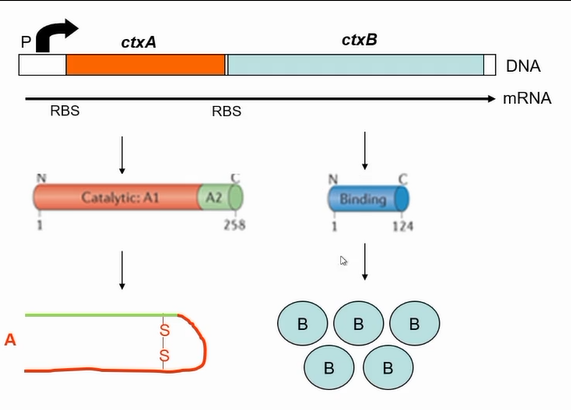
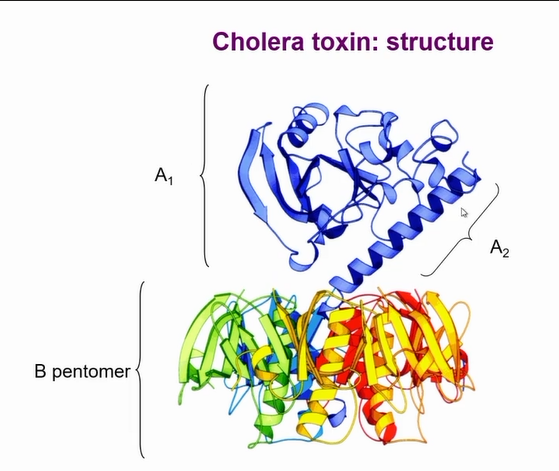
It is a complex AB toxin
Coded by two genes
Co-transcribed in a operon
ctxA and ctxB
Need more ctxB that ctxA
5-1 ctxB ratio to ctxA
More translation of B than a due to stronger ribosomal binding site
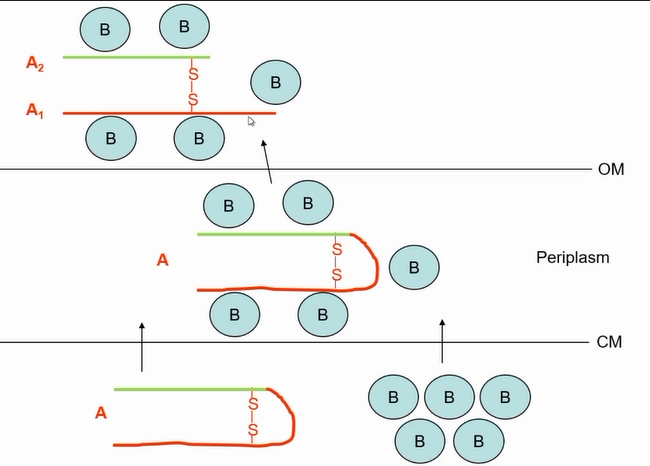
assembly of the cholera toxin
interaction of B to A
A1 is the catalytic part, A2 is responsible to tethering A to B
cholera toxin cell entry and action
Toxin is not endocytosed and the breaks out of endosome like normal AB toxins
Gets taken into a apical endosome by ganglioside
Gets taken to endoplasmic reticulum
B subunit still attached at this point
Then a isomerase breaks the disulphid bond that releases the A portion releasing the toxin from the endoplamic reticulum
Once in the cytosol acts on adenelate cyclase that is responsible for synthesisina cyclic AMP levels
A subunit nicked for activity; A1 translocated into host cell cytoplasm
A1 ADP ribosylates Gs (membrane protein) causing continual activation of adenylate cyclase
These are responsible for cell chloride secretion
Cholera toxin upregulates camp production resulting in more chloride loss and Na+ absorbtion
(via ADP-ribosolation)
whooping cough
Causative agent: Bordetella pertussis
Very small Gram –ve, aerobic, cocco-bacillus
respiratory metabolism, nutritionally fastidious
Transmission by aerosols or direct contact
Reservoirs: Infected humans esp. asymptomatic carriers
1st stage: resembles common cold
2nd stage: Dry cough which becomes paroxysmal,
excess mucus production & vomiting
Convulsions & cyanosis may occur
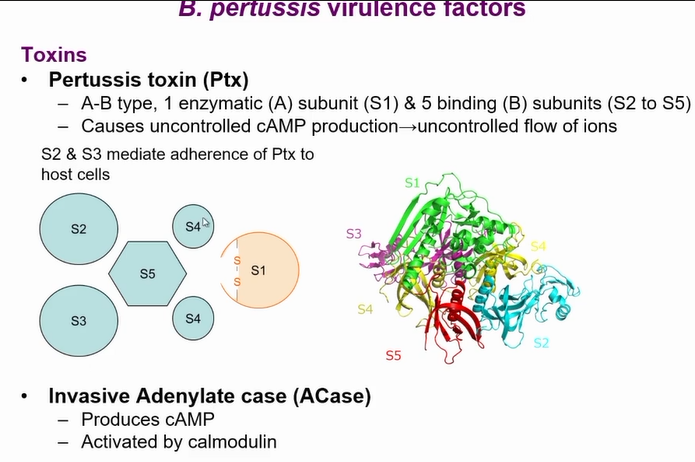
Virulence factors include pertussis toxin (Ptx) and ACase toxin
Prevention: antibiotics (don’t lessen duration of symptoms)
effective vaccine
resurgence of whooping cough
Rare thanks to vaccine but starting to make a come back particularly in Manchester
People stopped taking vaccine and started getting cough
Older people with the vaccine began to get a drop in immunity and caught it of unvaccinated people
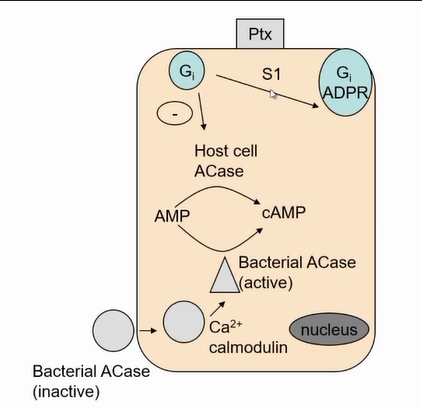
whooping cough pertussis toxin
Inactivates GI so it cant switch of active form of the enzyme results in exactly the same effect of cholera toxin
but acts in the airways
results in to much fluid being produced in the airways
toxins summary