Diabetes - anatomy, pathophysiology
1/75
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
76 Terms
What is the type of diabetes characterised by no insulin?
Type 1 diabetes
What is the type of diabetes characterised by an inability to respond to insulin as pancreas doesn’t make enough to meet the demand?
Type 2 diabetes
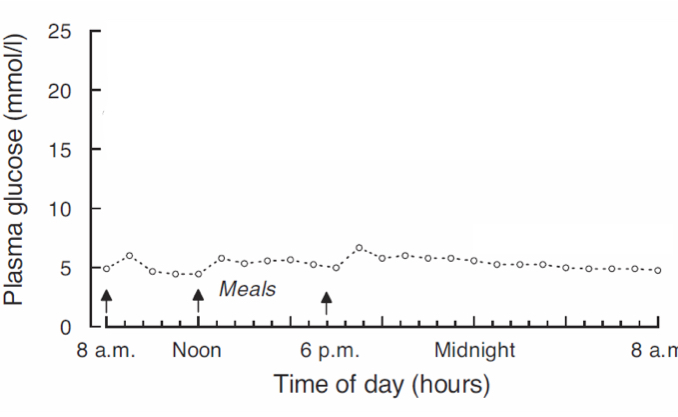
What is the usual blood glucose fluctuations in a person without diabetes?
Very little fluctuations, generally stays around 5mmol/L
What organs use the most energy?
Heart and kidney, followed by brain
What is the order of organs energy usage?
heart > kidney > brain. > liver > skeletal muscle > adipose tissue > residual
Why does skeletal muscle not use much energy?
Not using much energy usually but will increase if exercising e.g., at the gym, running a marathon
Why does the kidney require so much energy?
Uses lots of active transport to reabsorb ions
Where does active transport to reabsorb ions, glucose, amino acids and bicarbonate in the kidney occur?
Proximal tubule, loop of Henle, distal tubule, collecting duct
Where in the kidney does the majority of reabsorption of filtrate occur?
Proximal tubules
What is active transport powered by in the kidney?
Na+ K+ ATPase
What roles does Na+ K+ ATPase in the kidneys?
Establishes ion gradients and drives co-transporters
What does kidney reabsorption rely on/
Aerobic respiration - requires lots of blood supply
What energy source do the kidneys mostly use?
Beta oxidation of fatty acids e.g, palmitate producing 106 molecules of ATP
What area of the kidneys has one of the highest oxygen consumptions in the body?
Proximal tubules
What does the brain/neurons rely on for energy?
Glucose
How does a neuron use energy?
Maintaining resting potential
Propagation of action potential
Releasing neurotransmitter vesicles
Post-synaptic actions of neurotransmitters
Recycling neurotransmitters and vesicles
All of the above use ATPase pump
What can be signs of hypoglycaemia in type 1 diabetes?
Slurred speech, cognitive impairment, stumbling
Why does type 1 diabetes have cognitive symptoms in hypoglycaemia?
Brain cannot get the glucose it needs so stops working properly
Where does the majority of glucose in the blood come from?
Food or liver
What type of activity uses glucose/glycogen in muscles as an energy source?
Anaerobic e.g., sprinting
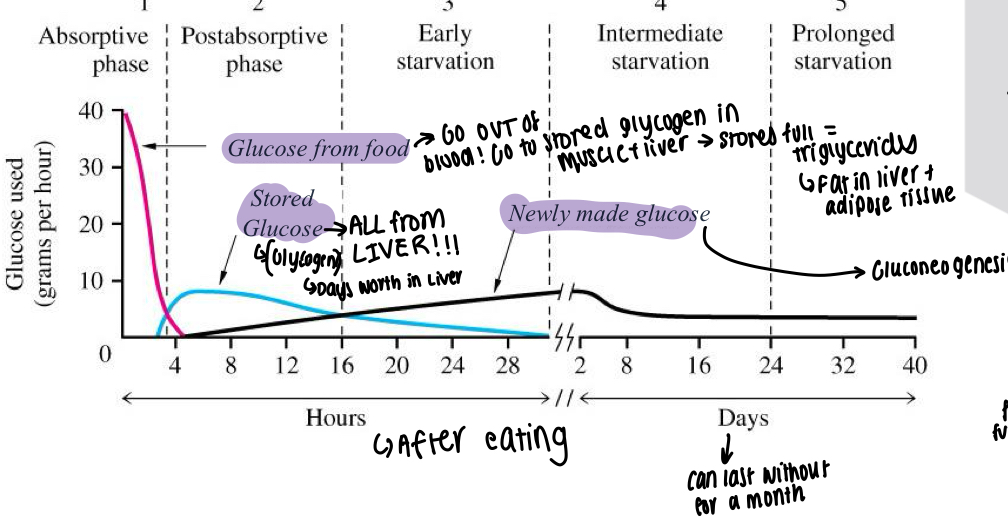
What is the graph showing glucose usage after eating and the different stages?
Glucose from food used for the first few hours, is also being moved into muscle and liver to be stored as glycogen
If stores are full, triglycerides made and put into liver and adipose tissue
At around 4 hours - stored glucose from the liver begins to work, about a days worth of
Newly made glucose begins to take over at 24 hour mark, Gluconeogenesis occurs and can keep going for days-weeks
In starvation, where is the glucose from for the brain to use?
Uses fat initially, then carbon for glucose from muscle cells amino acids
In general, what energy source will organs use?
Brain = always glucose
Kidney = fatty acids
Those that switch will e.g., if more glucose, will use glucose
What organ tops up glucose in between meals?
Liver
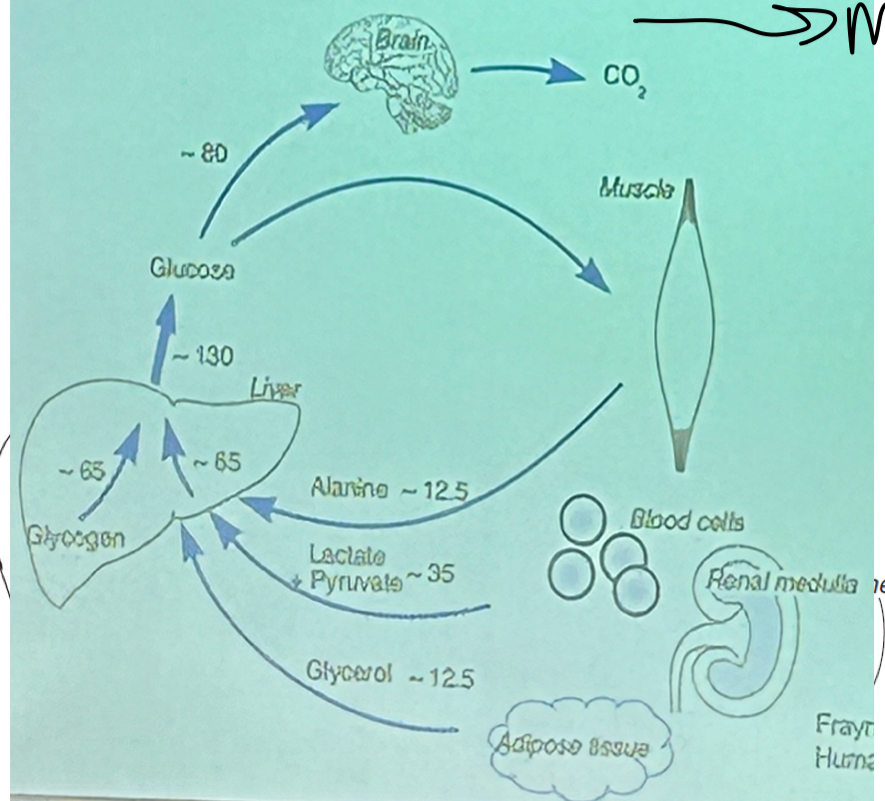
What is a diagram showing the glucose flux after an overnight fats?
Most diverted towards the brain
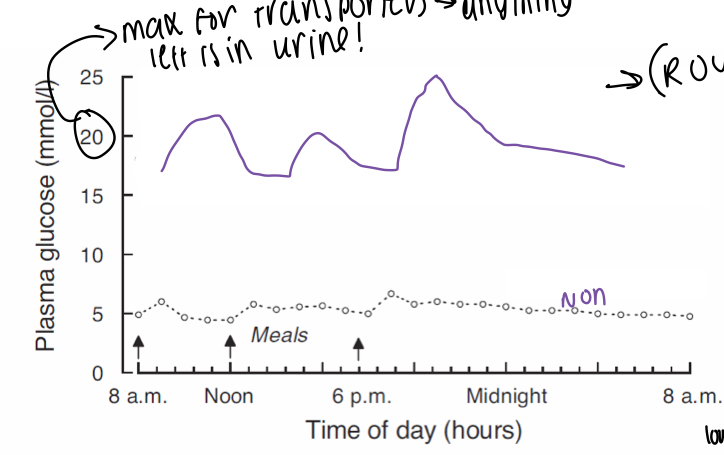
What is the graph showing the blood glucose variations in a non-diabetic vs a diabetic?
Much higher, wider variations after a meal, maximum for transporters is around 20 so may have lots of glucose left in the urine
What effect do SGLT2 drugs have due to making the body absorb less glucose?
Urinate more frequently
What is a side effect of SGLT2 drugs making patients urinate more?
Increase susceptibility to lower UTI infections
What is the term to describe the metabolic disease characterised by raised plasma glucose levels?
Diabetes
What is diabetes caused by?
Failure of insulin and regulation of metabolism, can switch off fatty acid release from fat stores also
What are the 2 main complications from hyperglycaemia?
Microvascular and macrovascular
What are examples of microvascular damage caused by hyperglycaemia?
Blindness, limb amputations, kidney failure
What are examples of macrovascular damage caused by hyperglycaemia?
Heart attacks, strokes
Why does microvascular complications occur?
Damage to endothelial lining of small blood vessels
What are the 3 mechanisms for endothelial damage?
Sorbitol production using NADPH which increases oxidative stress and reduces vasoelasticity
Glycation of proteins alters cellular interactions and extracellular matrix
Acetyl CoA makes fatty acids which makes diacyl glycerol - signalling molecule which alters cellular interactions signalling
How do macrovascular complications occur from hyperglycaemia?
increases free fatty acids, leading to plaque deposition in arterial walls and increases FAOx which can lead to oxidative stress
What diseases is diabetes an independent risk factor for?
Coronary artery disease, cerebrovascular disease, peripheral vascular disease
What can diabetes be a cormorbid risk factor for?
Obesity, hypertension, hyperlipidaemia, altered platelet function
When does macrovascular disease present in type 2 diabetes?
At diagnosis
When does macrovascular disease present in type 1 diabetes?
Age and duration of diabetes correlates with degree
What do most treatments for diabetes target?
Hyperglycaemia
Why is glucose used as a target in diabetes?
Easily measurable, key to target
What is a diagram comparing type 1 and 2 diabetes?
T1 is early onset, type 2 is later
Lean physique in type 1, obese in type 2
Treatment for type 1 is insulin, type 2 is diet, drugs and insulin
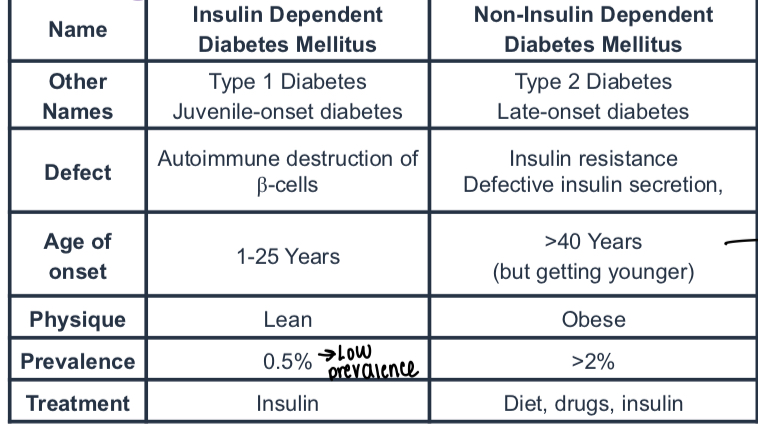
What is the defect in type 1 diabetes?
Autoimmune destruction of Beta cells
What is the main defect in type 2 diabetes?
Insulin resistance or defective insulin secretion
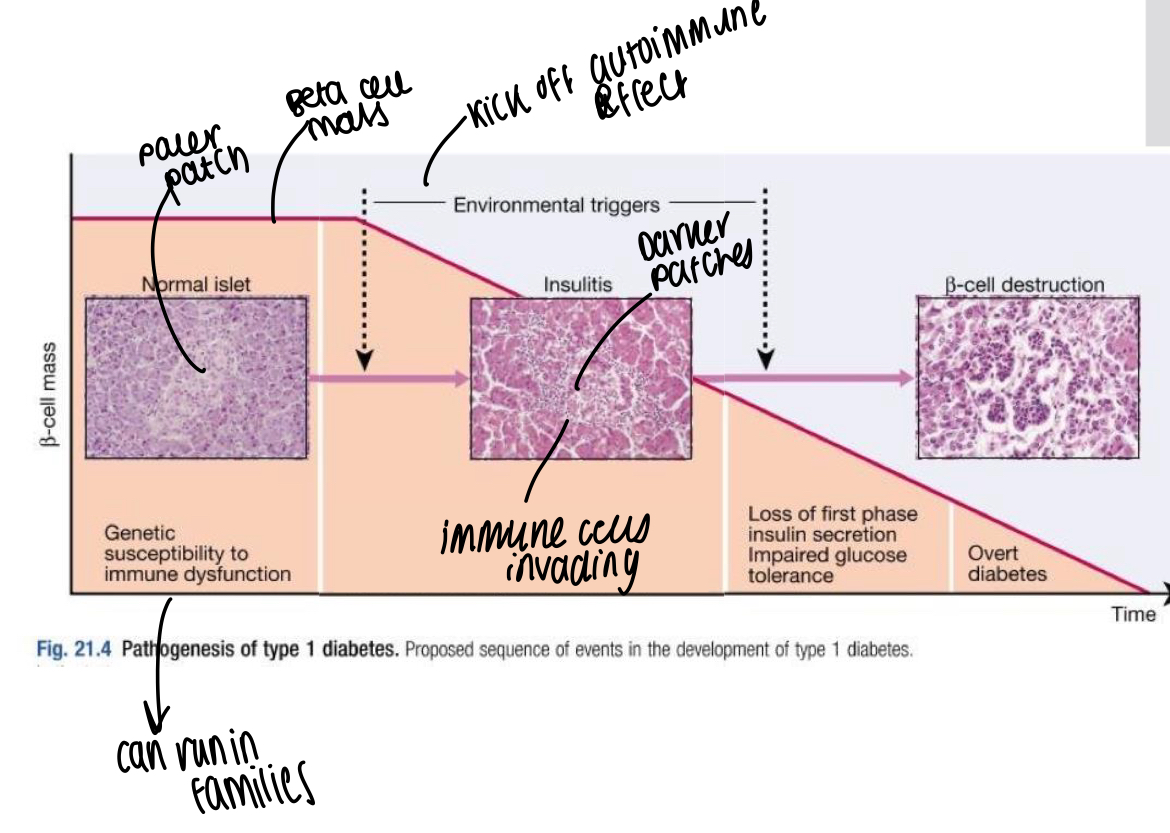
How does the autoimmune response in type 1 diabetes start?
Antibody mediated immune destruction of beta cells from islets of Langerhans, progressive loss occurs over months and years and usually appeared in adolescence where hyperglycaemia noticed when 80-90% of beta cells lost
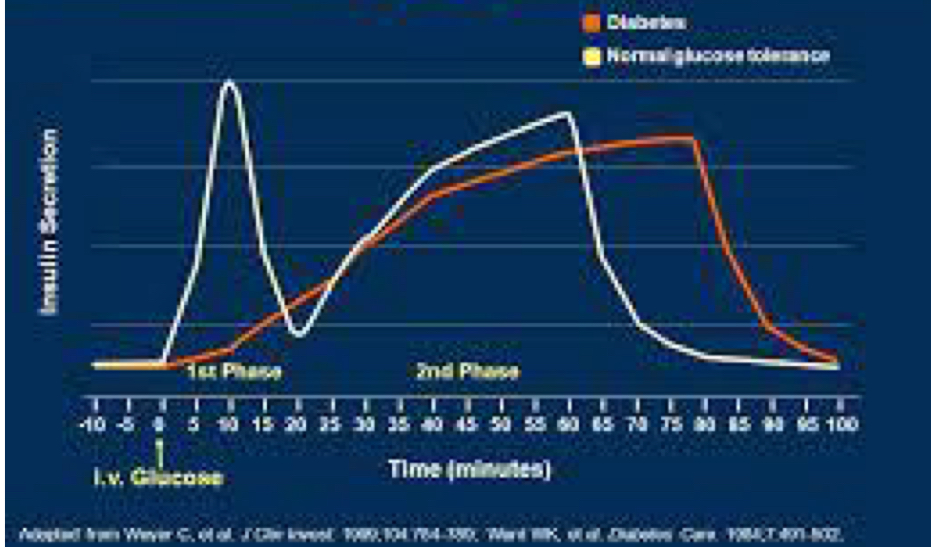
What is post-meal insulin release like in type 1 diabetes?
Spike occurs a lot later than usual
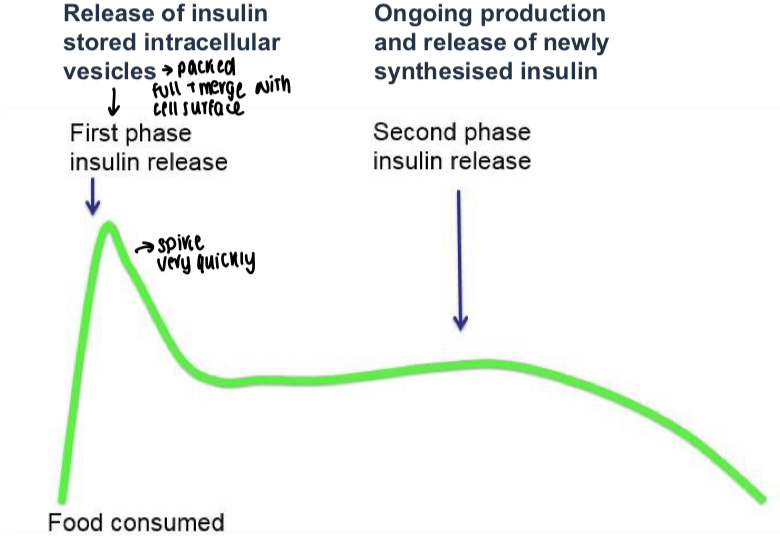
What is the usual graph showing post-meal insulin release?
Stable first peak that spikes quickly before second phase release
What accounts for 1/3 of susceptibility for type 1 diabetes?
Genetic factors
What are some candidates for environmental factors affecting type 1 diabetes?
Viruses - mumps, rubella, retroviruses
Specific drugs or chemicals - specific nitrosamines in smoked and cured meats, coffee
Dietary constituents - cow milk in infancy
Reduced exposure to microorganisms in early childhood
What are some major complications of type 1 diabetes?
Chronic hyperglycaemia effects
Hypoglycaemia from over administering insulin
Diabetes ketoacidosis
What can be a cause of hypoglycaemia in type 1 diabetes?
Patient over administering too much insulin - an acute state
What is the term to describe uncontrolled glucose and fatty acid oxidation levels in diabetes, where ketone bodies enter the brain in diabetes?
Diabetes ketoacidosis
What is the issue with ketone bodies entering the brain in diabetic ketoacidosis?
NOT needed in the brain and can make the blood very acidic, leading to coma and death
What is the diagram showing usual usage of glucose in the fasting state in an individual WITHOUT diabetes?
Low plasma glucose levels, Triglycerides broke down and used in liver to make glucose, Gluconeogenesis also occurs
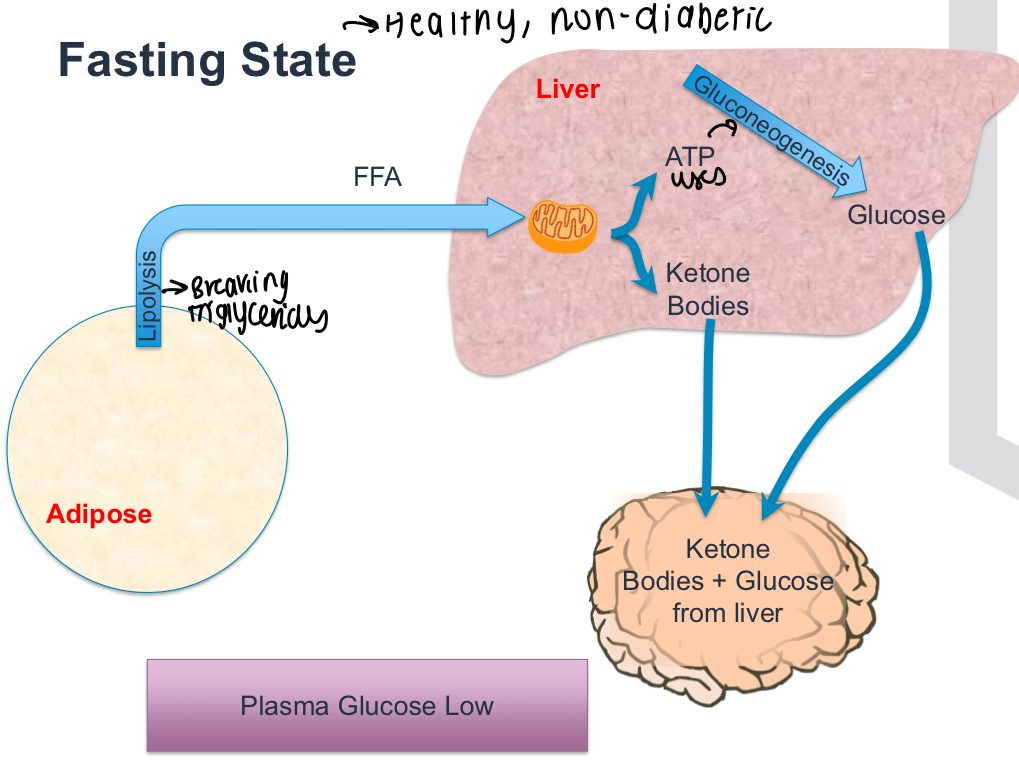
What happens in the fed state in a normal person without diabetes?
No lipolysis occurs, insulin from pancreas switches off Gluconeogenesis etc and reduces plasma glucose levels
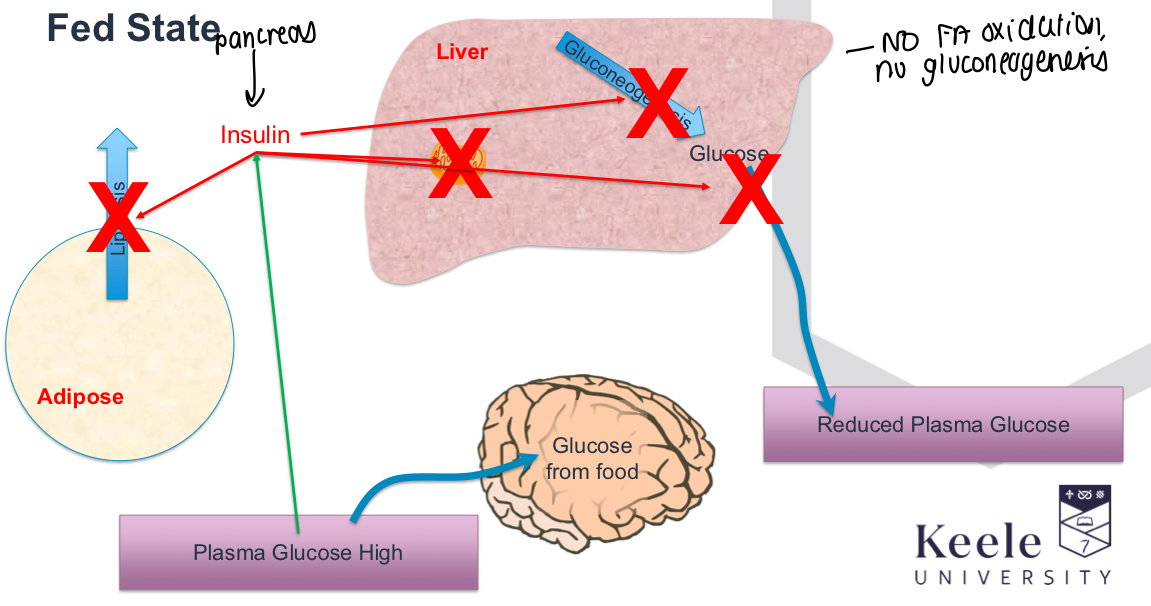
What happens in high plasma glucose levels/fed state in diabetes?
Insulin doesn’t work, lipolysis uncontrolled and keeps going, plasma glucose and ketone bodies remain high, Gluconeogenesis keeps going
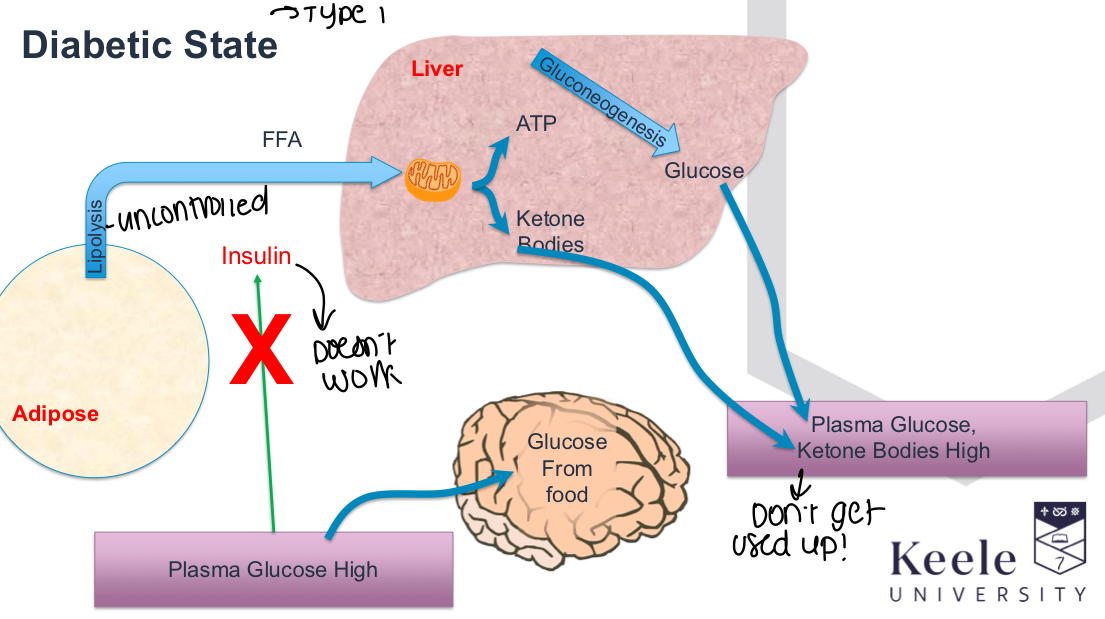
What processes are uncontrolled that lead to diabetic ketoacidosis?
Lipolysis and beta oxidation, also high blood glucose
What causes type 2 diabetes?
Hyperglycaemia and high free fatty acids, glucose unable to exert effects on liver and adipose
What happens to beta cells in type 2 diabetes?
Produce more insulin to try and overcome insulin resistance, until they are overwhelmed and insulin cannot switch off
What causes insulin resistance in type 2 diabetes?
Strong genetic components e.g., in twins and in indigenous populations
Environment is key!! Lifestyle, exercise and DIET
What are some reasons that cause insulin resistance?
Ectopic lipid accumulation, cellular stress responses, inflammation
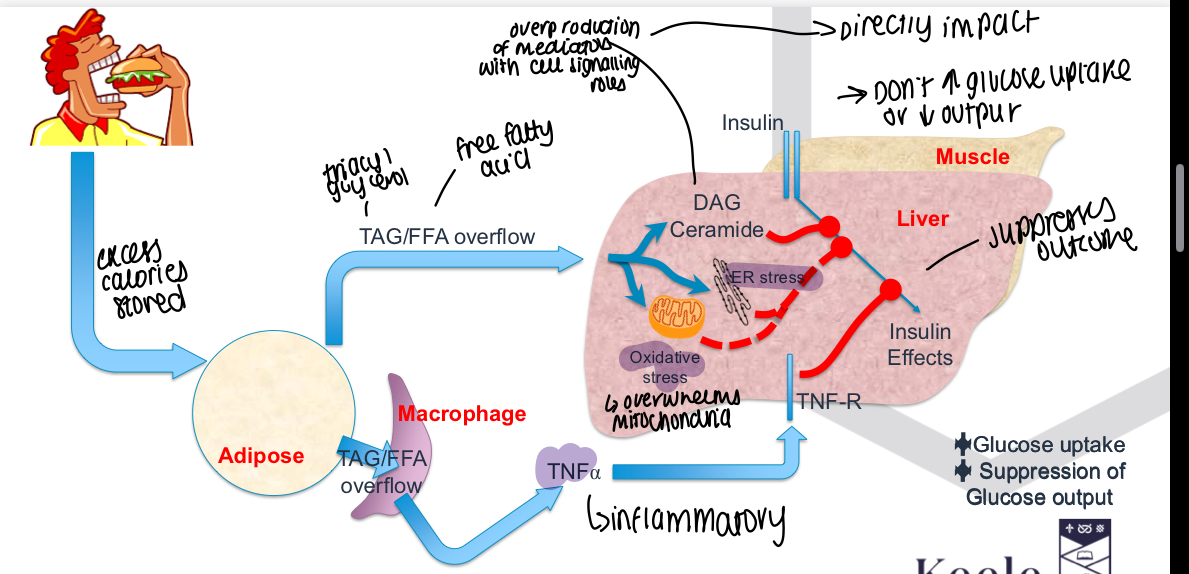
How does ectopic lipid accumulation cause insulin resistance?
Lipids in the wrong place - should usually be in adipose tissue, liver and muscle triglycerides have less insulin sensitivity, accumulates lipid mediators and can alter protein phosphorylation
How do cellular stress responses cause insulin resistance?
Mitochondrial and protein producing machinery break down, alters insulin signalling pathways - downstream of ectopic lipid accumulation
How does inflammation cause insulin resistance?
Macrophages in adipose tissue accumulate in lipid, when it becomes too full leaks triglycerides into surrounding tissue and secretes inflammatory cytokines such as TNF alpha and alters insulin signalling pathways in muscle and liver
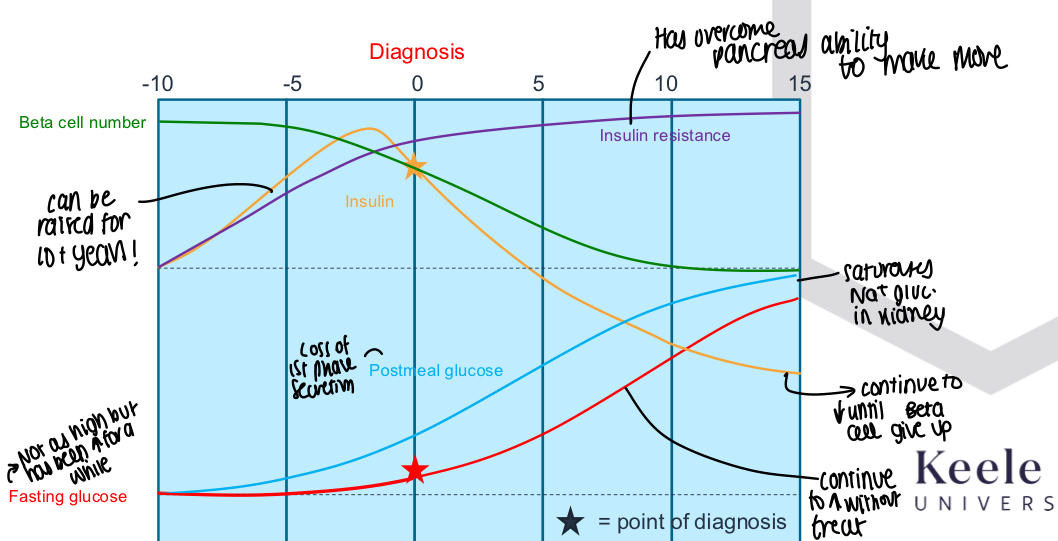
What is a graph showing history of type 2 diabetes before/after diagnosis?
Insulin can be raised for years before dipping
Beta cell number slowly decreases
Fasting glucose and postmeal glucose both steadily rise
Insulin resistance increases over time
What can microvascular damage lead to in diabetes?
Retina, kidney and nerve damage
What does diabetic retinopathy lead to ultimately?
Blindness
Related to the retina, what are diabetic patients offered more frequently?
Free eye tests
What can diabetic nephropathy lead to?
End stage renal failure
What can diabetic neuropathy lead to?
Debilitating neuropathy and lead to diabetic foot
What is peripheral neuropathy caused by?
Endothelial damage, wall thickening leads to ischaemia and neural death, segmental demylinisation and slowing of nerve conduction occurs
What are the.2 different types of diabetic peripheral neuropathies?
Somatic and autonomic
What are the somatic symptoms of diabetic peripheral neuropathies?
Parathesias, including numbness and tingling, impaired pain, termpature, light touch, two-point discrimination and vibratory sensation
What are autonomic symptoms of diabetic peripheral neuropathies?
Vasomotor functions e.g., postural hypotension
GI function - gastric atony, postprandial and nocturnal diarrhoea
Genitourinary function - Paralytic bladder/incomplete voiding, impotence
Cranial nerve - impaired pupillary response s
What can patients be unaware of. For foot problems in diabetic foot ulcers?
Poorly fitting shoes, improper weight baring or iinfections