Chapter 5: Protein Function
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
globular proteins functions
storage of ions/molecules (myoglobin, ferritin)
transport of ions/molecules (hemoglobin, serotonin transporter)
defense against pathogens (binding antibodies, cytokines)
muscle contraction (actin, myosin)
catalysis (enzymes)
ALL bind something and let it go (so a reversible process)
the binding of molecules to proteins is
reversible
ex: ligands can bind and unbind at the binding site of a protein
ligands bind via
the same NON-covalent forces that dictate protein structure
so therefore are reversible
cooperativity
when there are more than one binding site on a protein so the binding of a ligand to one can influence the next subunit
ex: hemoglobin
factors that dictate how a ligand binds
size
shape
charge
hydrophobicity
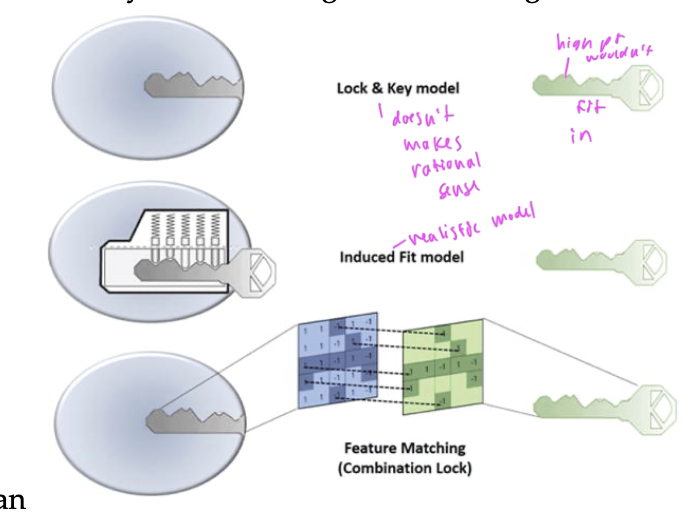
two models of ligand binding
lock and key model: assumes that a ligand can perfectly fit into a protein without any conformation change (not true)
induced fit model: where both the protein and ligand make conformational changes so the binding site fits the ligand better
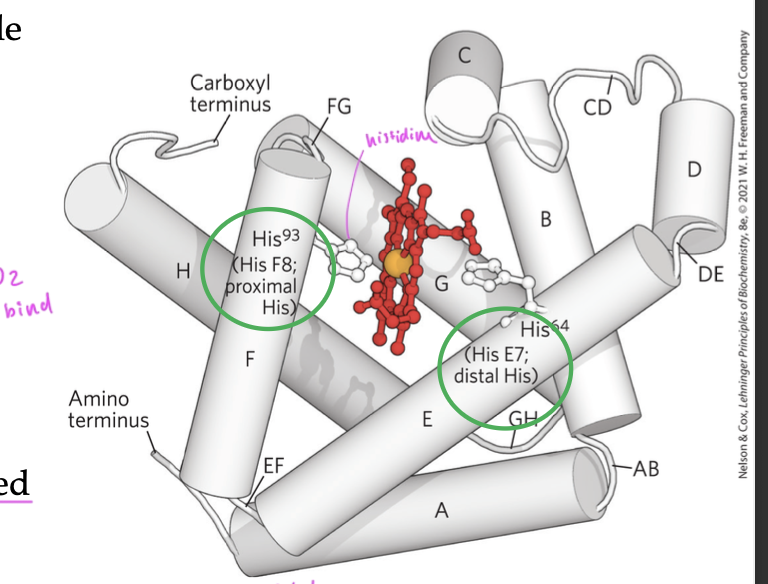
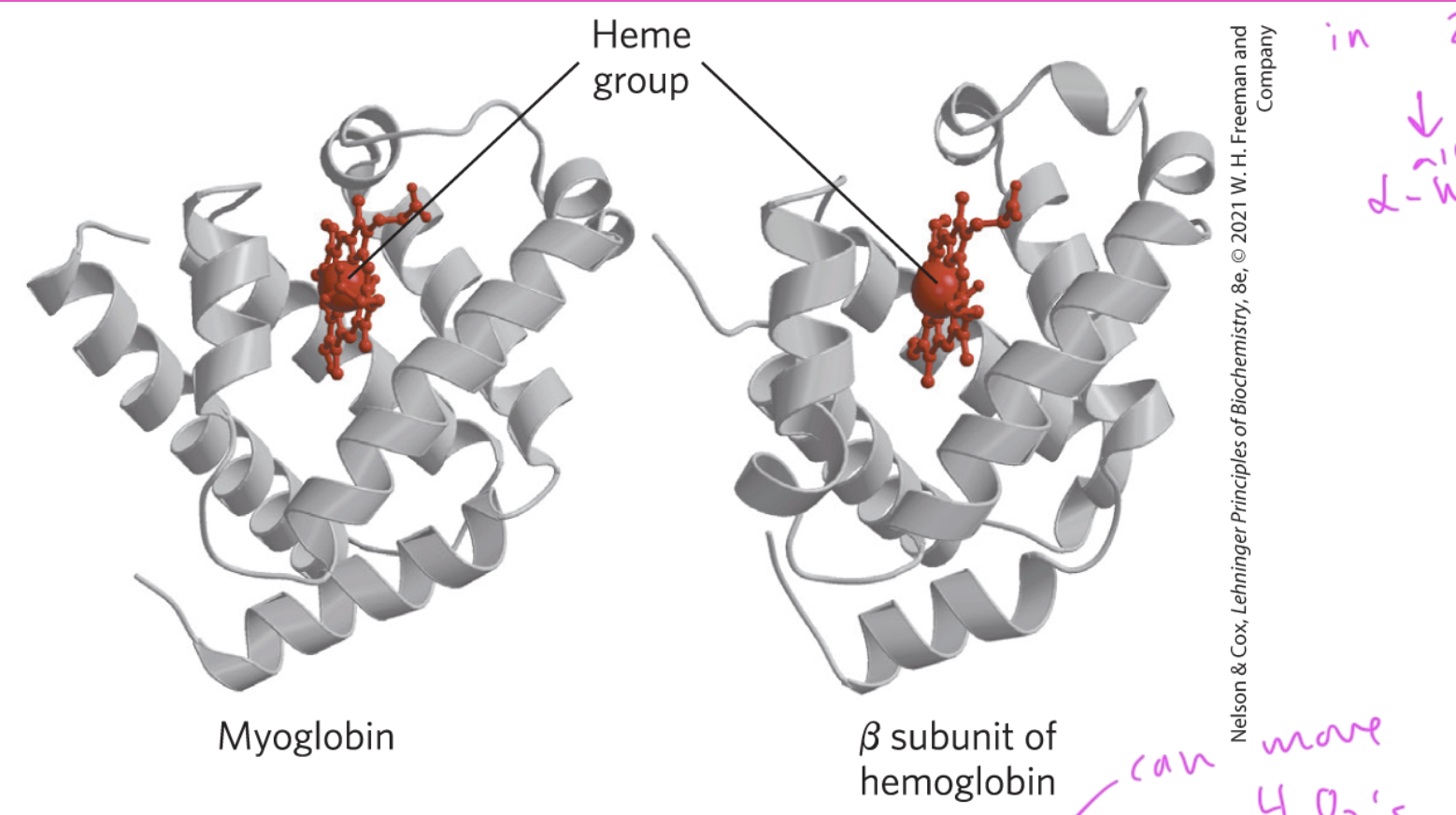
myoglobin (Mb) contains which secondary structures
only alpha helices
myoglobin is a single
polypeptide chain
made of 8 alpha helices (A—>H)
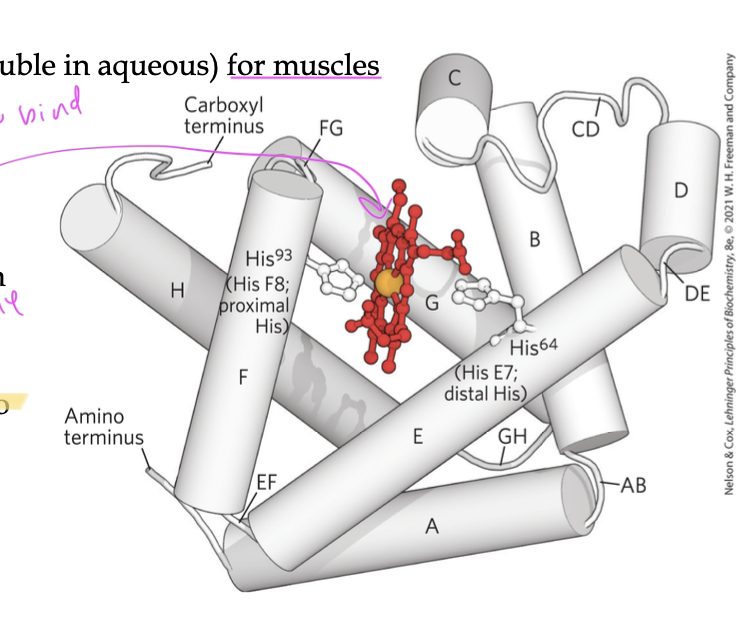
function of myoglobin
carries and stores oxygen using its heme group (a porphyrin ring with Fe2+)
O2 binds to the Fe2+
Why do you need the heme group for myoglobin to bind oxygen?
since the amino acids of the myoglobin can’t bind oxygen on its own
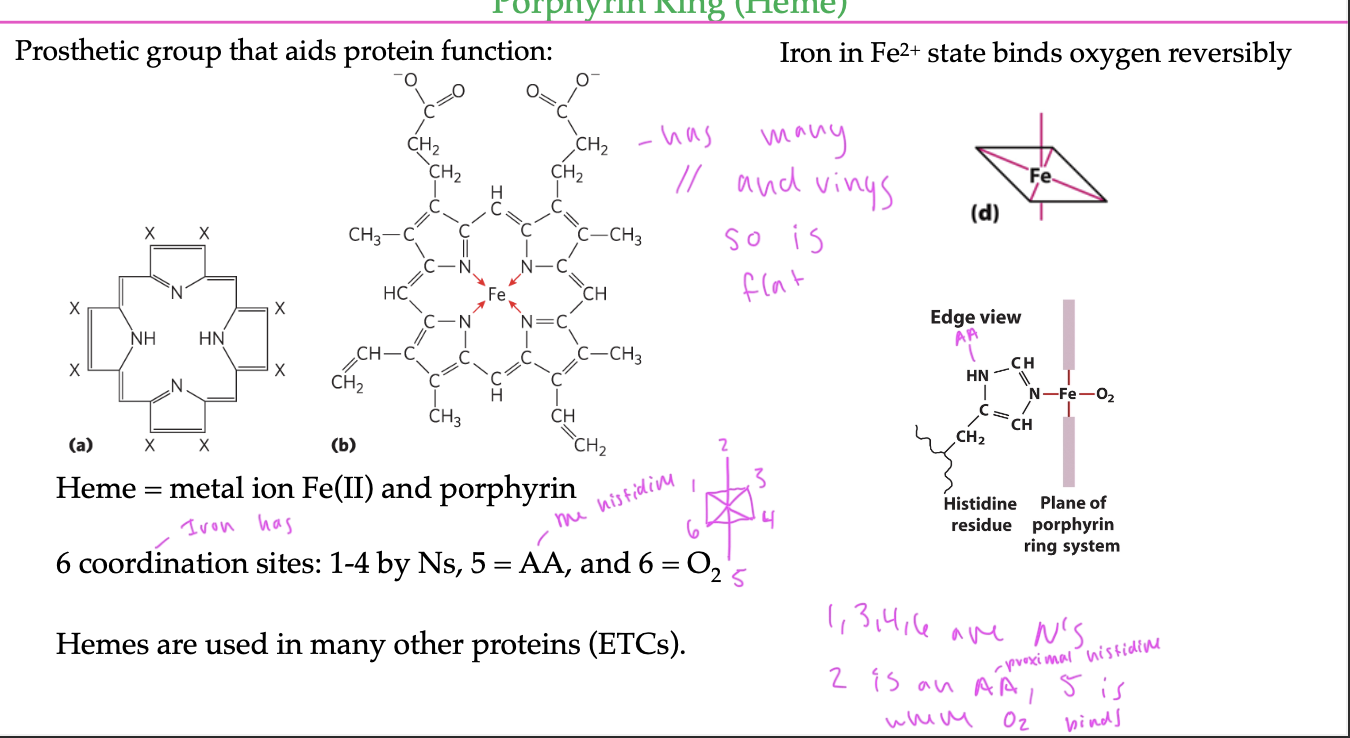
porphyrin ring
a prosthetic group that enables the Fe2+ of it to bind to oxygen in a myoglobin protein
has many c=c bonds and rings, so is flat/2D
has 6 coordination sites off the Fe2+
4 of which bind to N atoms
1 binds to oxygen
1 binds to the histidine residue
the Mb protects the iron atom from
oxidation
if it oxidizes, it becomes a Fe3+ ion, a superoxide ion
oxygen will only bind to Fe2+ so you want to stop the iron from oxidizing
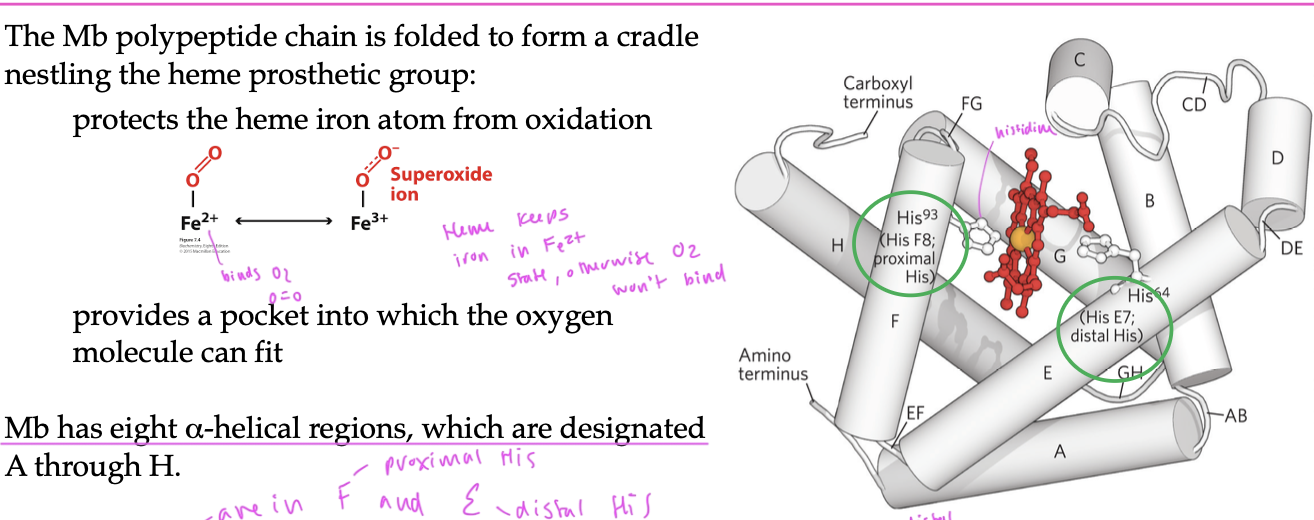
histidine residues in Mb
two polar His residues interact with the heme group
His E7 (distal) sterically inhibits CO from binding to the heme and controls the shape of the O2 binding site so that the O2 does not bind bind perpendicularily
since CO also wants to bind to the Fe 2+ of the heme, His E7 forces ligands to bind at an angle, so that O2 can bind better than CO (even though CO binds much better to a free heme)
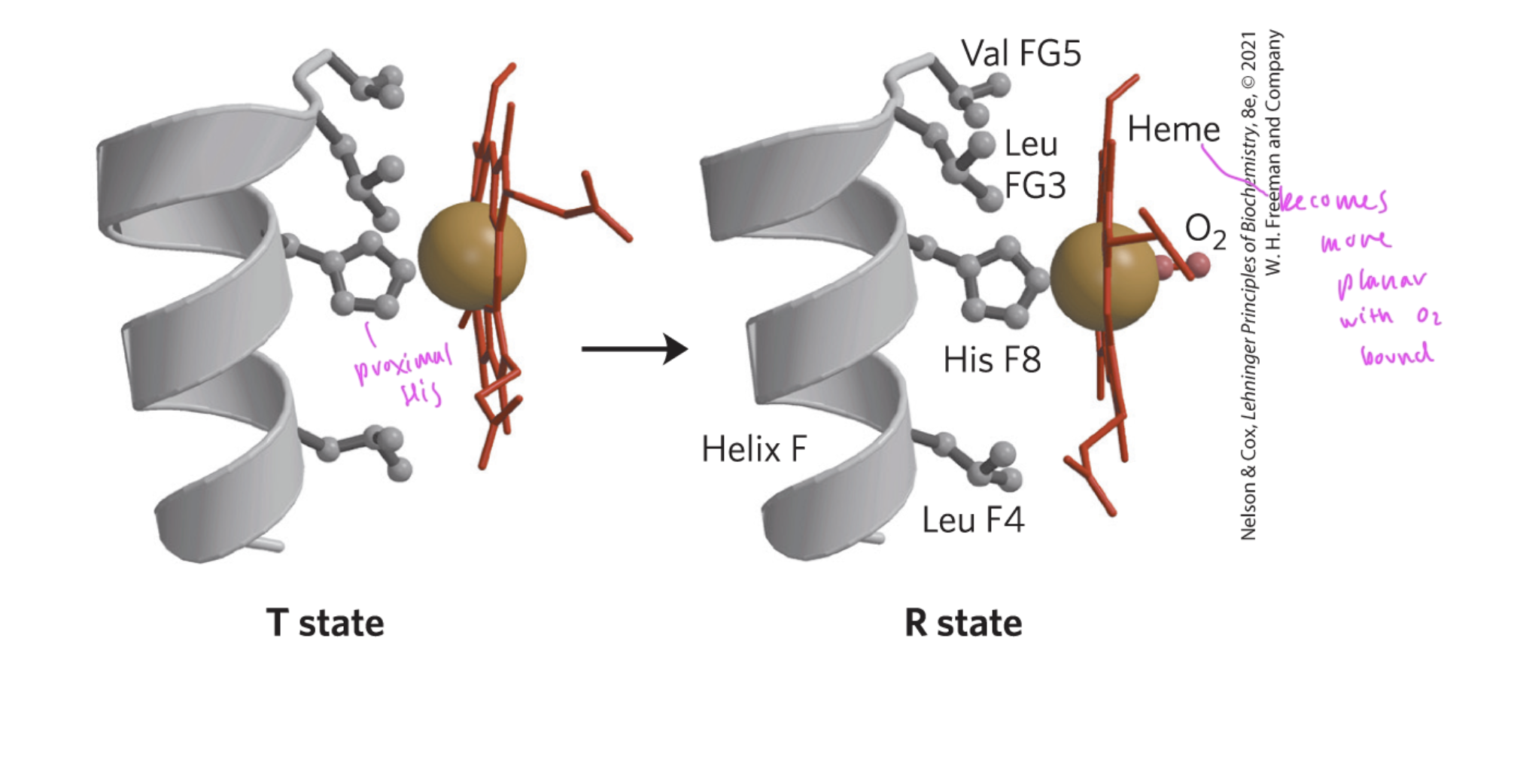
His F8 (proximal) always bind to one coordination site of the heme group
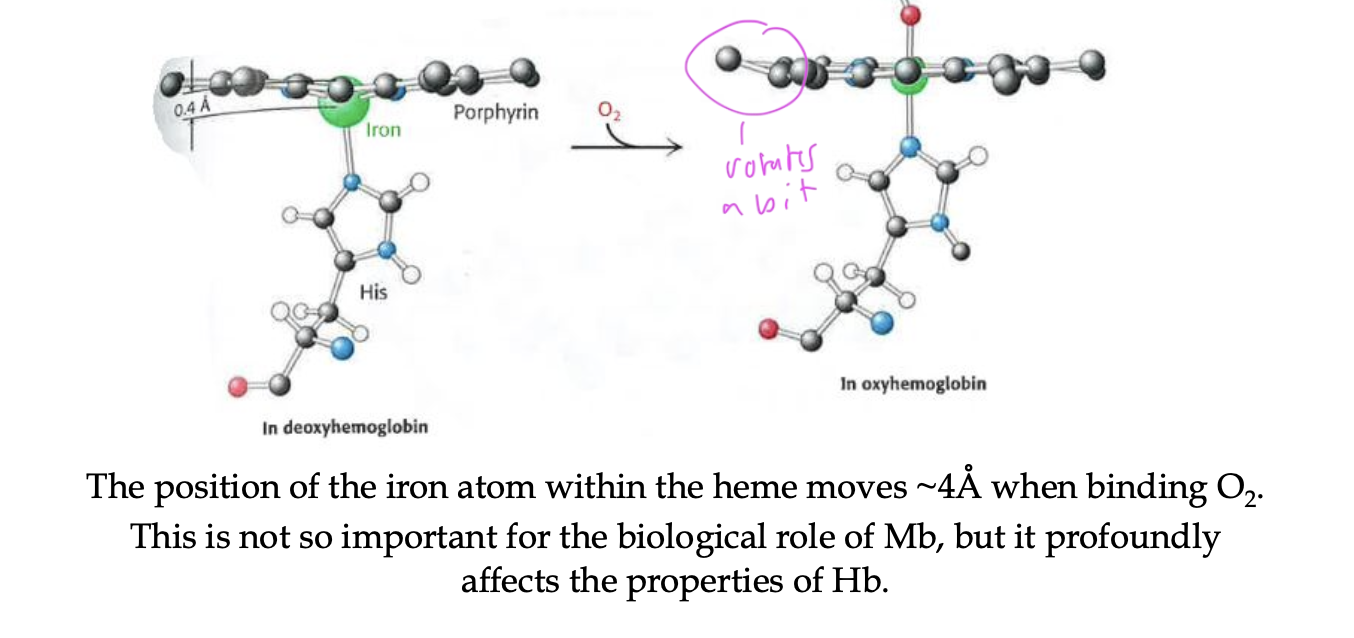
how does the binding of O2 affect Mb/Hb structure
the Fe2+ atom within the heme moves a tiny bit
affects the properties of Hb more than Mb
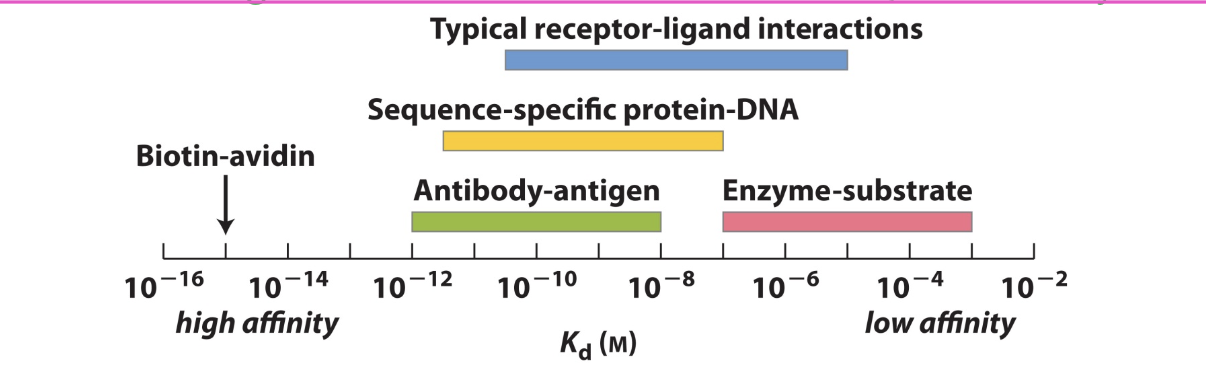
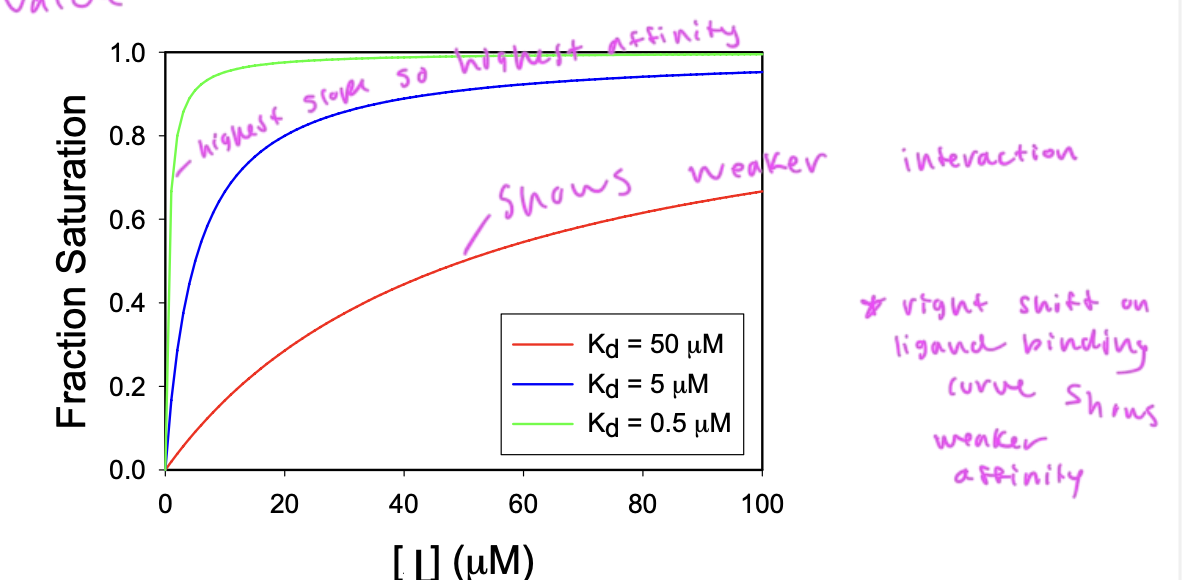
a lower Kd shows
higher affinity
since Kd is the dissociation constant
kd=
[P][L]/[PL]
so higher Kd corresponds to more dissociation being favored
Kd is the conc of ligand at which ½ of the available ligand binding sites are filled
saturation
when ligand binding reaches a maximum
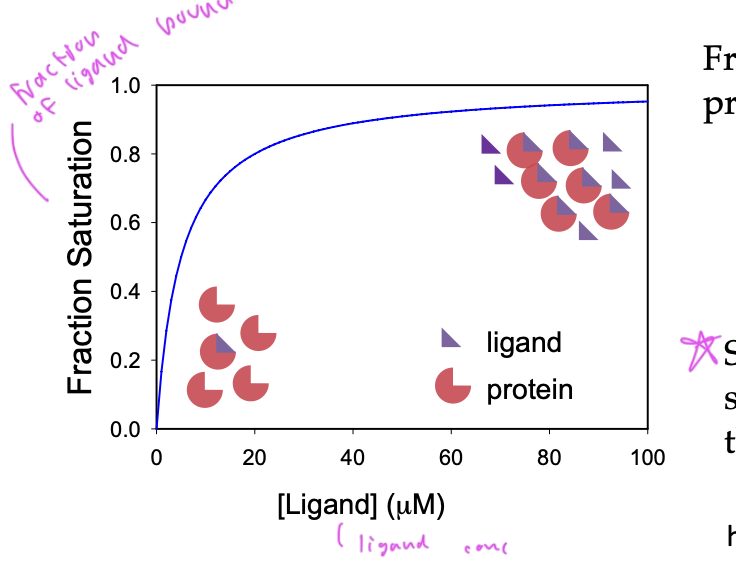
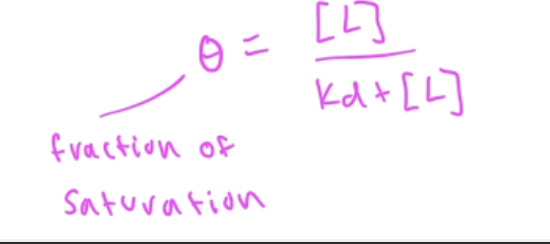
fraction saturation
Y=fraction saturation=#occupied binding sites/total binding sites
slope of the curve (how quickly you reach saturation) depends upon the affinity of the interaction
Y=[PL]/[Ptotal]
a steep slope shows higher affinity, and lower Kd
binding affinity
is shown by the Kd value
[L]=Kd, then half of the proteins are bound to ligands
[L] >Kd then most ligands are bound to proteins
[L}<Kd then most ligands are not bound to proteins
![<p>is shown by the Kd value</p><p>[L]=Kd, then half of the proteins are bound to ligands</p><p>[L] >Kd then most ligands are bound to proteins</p><p>[L}<Kd then most ligands are not bound to proteins</p>](https://knowt-user-attachments.s3.amazonaws.com/af1d58df-02a8-41c6-b998-b324795014c8.png)
ligand binding curves
shows what fraction of ligands are bound, so basically at what fraction of total saturation you are at based on the amount of ligand
saturation always =1.0
Kd is the value when: you go to half of the total/peak saturation and divide that value by two. Find that value on the y-axis on the function then go straight down and see at what conc of ligand on the x-axis you have
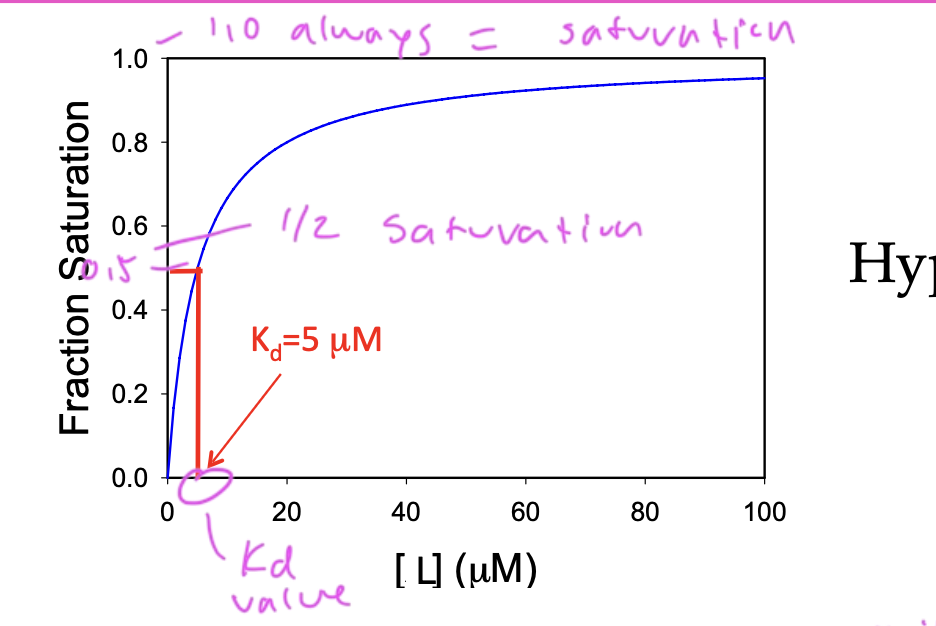
on a ligand binding curve, if [L]=0, fraction saturation (theta) equals
0
on a ligand binding curve, if [L]>Kd then fraction saturation (theta) equals
1
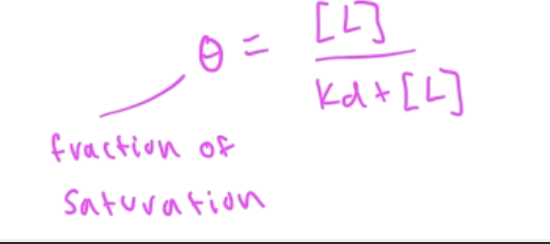
on a ligand binding curve, if [L]=Kd, then fraction saturation (theta) equals
0.5
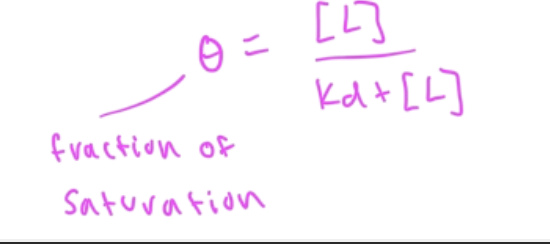
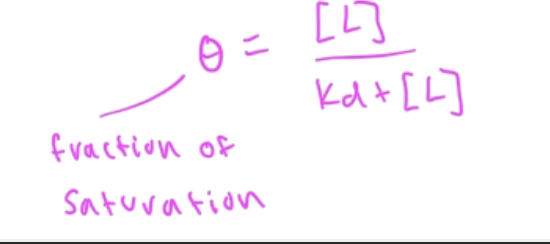
fraction of saturation (theta) eq
a shift right on a ligand binding curve means
the ligand has weaker affinity
and vice versa
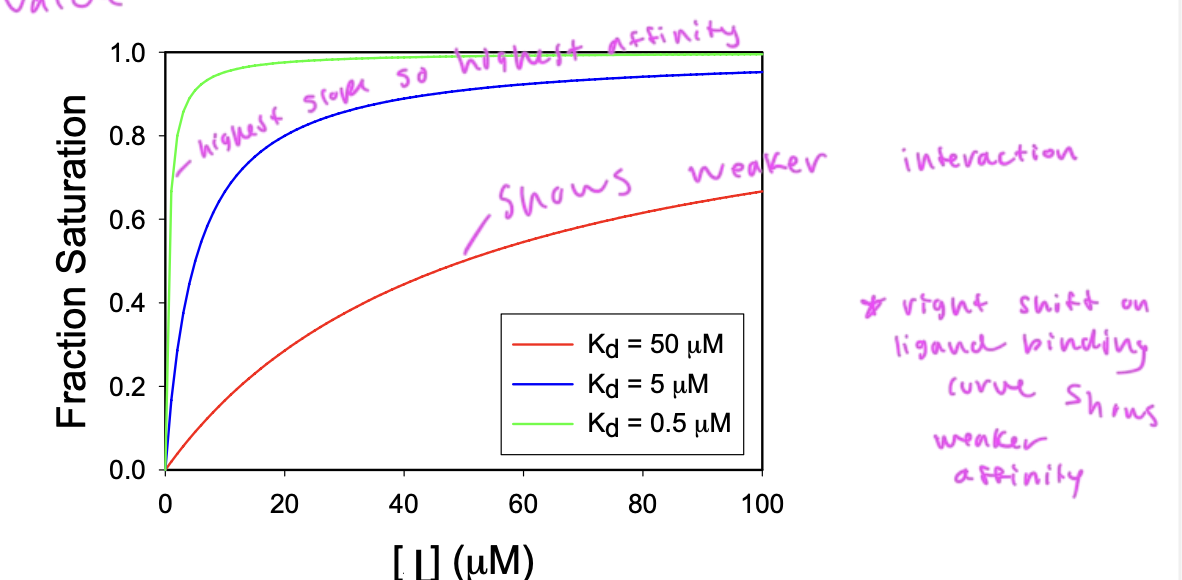
a higher slope on a ligand binding/fraction saturation curve shows
the extent of a ligand’s affinity for its substrate
so Kd decr as binding affinity incr
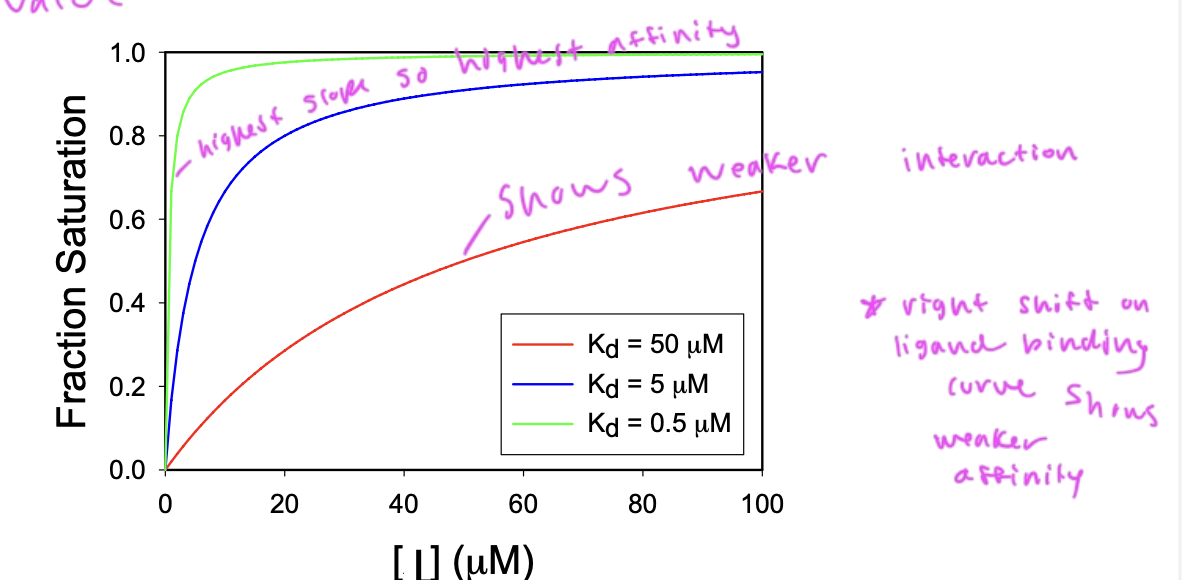
what type of ligand binding curve would you have for 1 protein type and 1 ligand type
a hyperbolic curve
myoglobin binds better in the lungs than
in the tissues
pO2 in lungs is 13kPa and is 4 pKa in tissues
so really, Mb is not a good deliverer of oxygen in the body since it has such a high affinity for the oxygen and won’t release it very easily
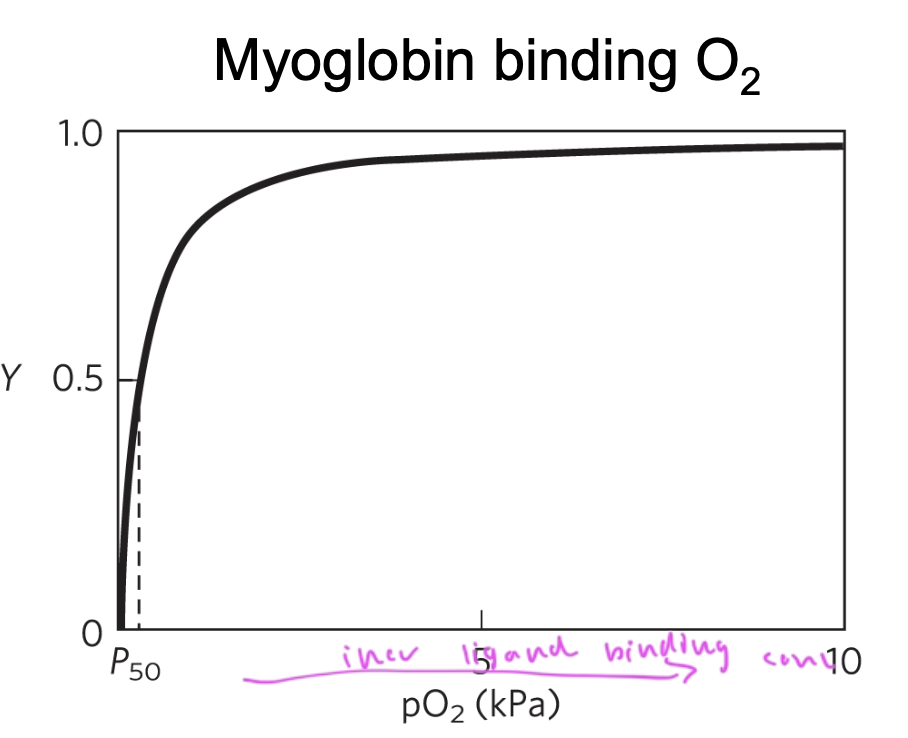
when does Mb release O2?
oxygen is stored in the body by Mb and Mb only releases it when muscles have very low oxygen due to exertion and need it
so Mb is not an effector deliverer of oxygen to the body as it just wants to hold onto the O2
agonist
a compound that causes a physiological response
is activating
antagonist
binds so that an agonist cannot
interferes with/deactivates a physiological action of another compound
you want drugs that
have a high affinity for their substrate
since you want the ligand to go to its substrate
so you would want a ligand binding curve that has a left shift (high affinity to the binding site)
Hb (hemoglobin) has __ O2 binding sites
4 since it has 4 separate Hb proteins in it
2 alpha subunits and 2 beta subunits
myoglobin has ____ subunit(s)
1
so only stores 1 O2 molecule
and only has 1 heme group
hemoglobin has ____ subunit(s)
4
so has 4 heme groups, which can each store an oxygen
Mb and Hb have very similar
secondary structure
are both just made up of a bunch of alpha helices (with the heme group ofc)
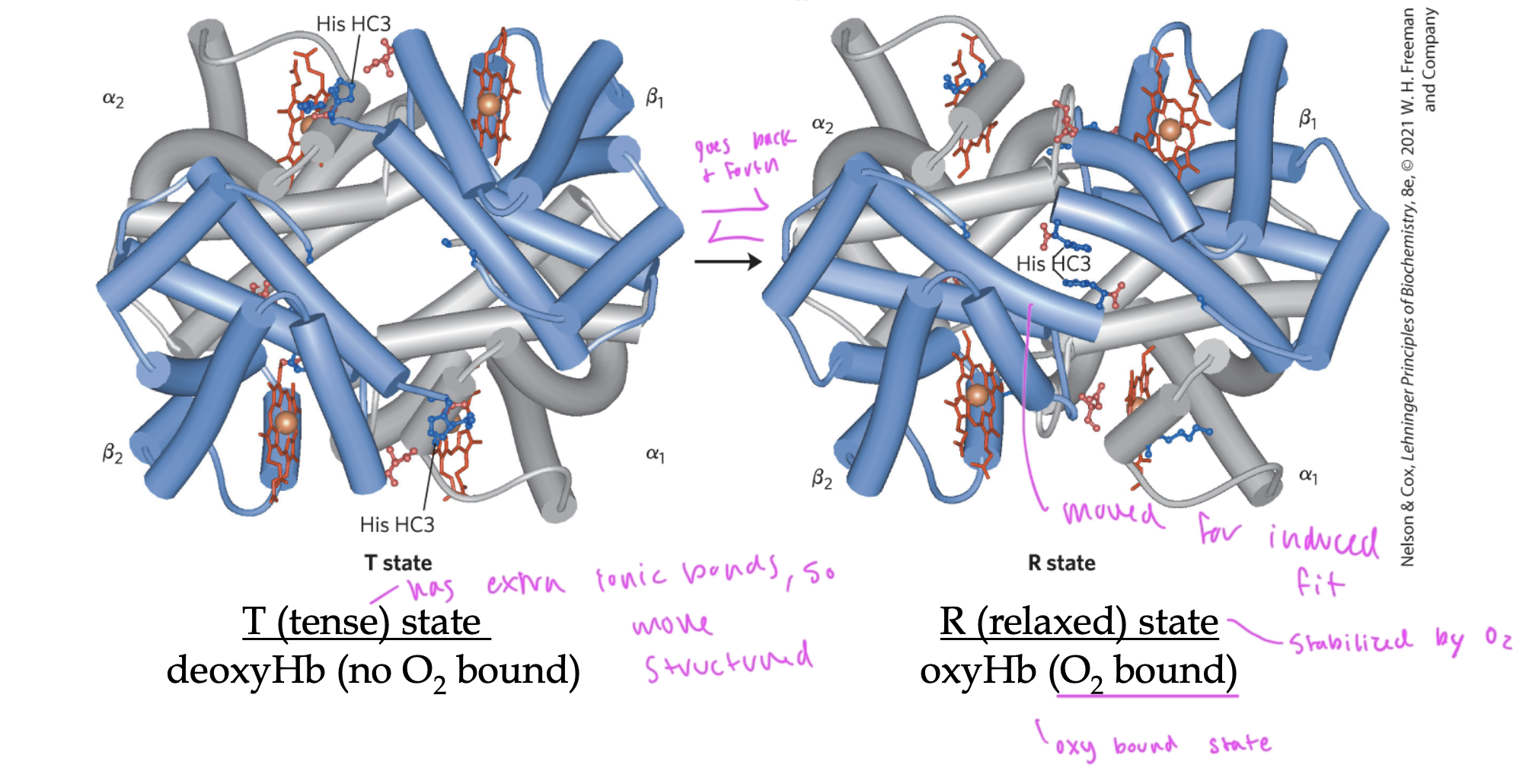
T state of Hb
deoxyHb (no O2)
“tense” state
has extra ionic bonds, so is more structured
does not usually bind O2 since it does not have induced fit for the oxygen
R state of Hb
oxyHb (has O2)
“relaxed state”
lacks that extra structure that T state has, so can conform to fit the oxygen in it by doing induced fit, so holds an O2
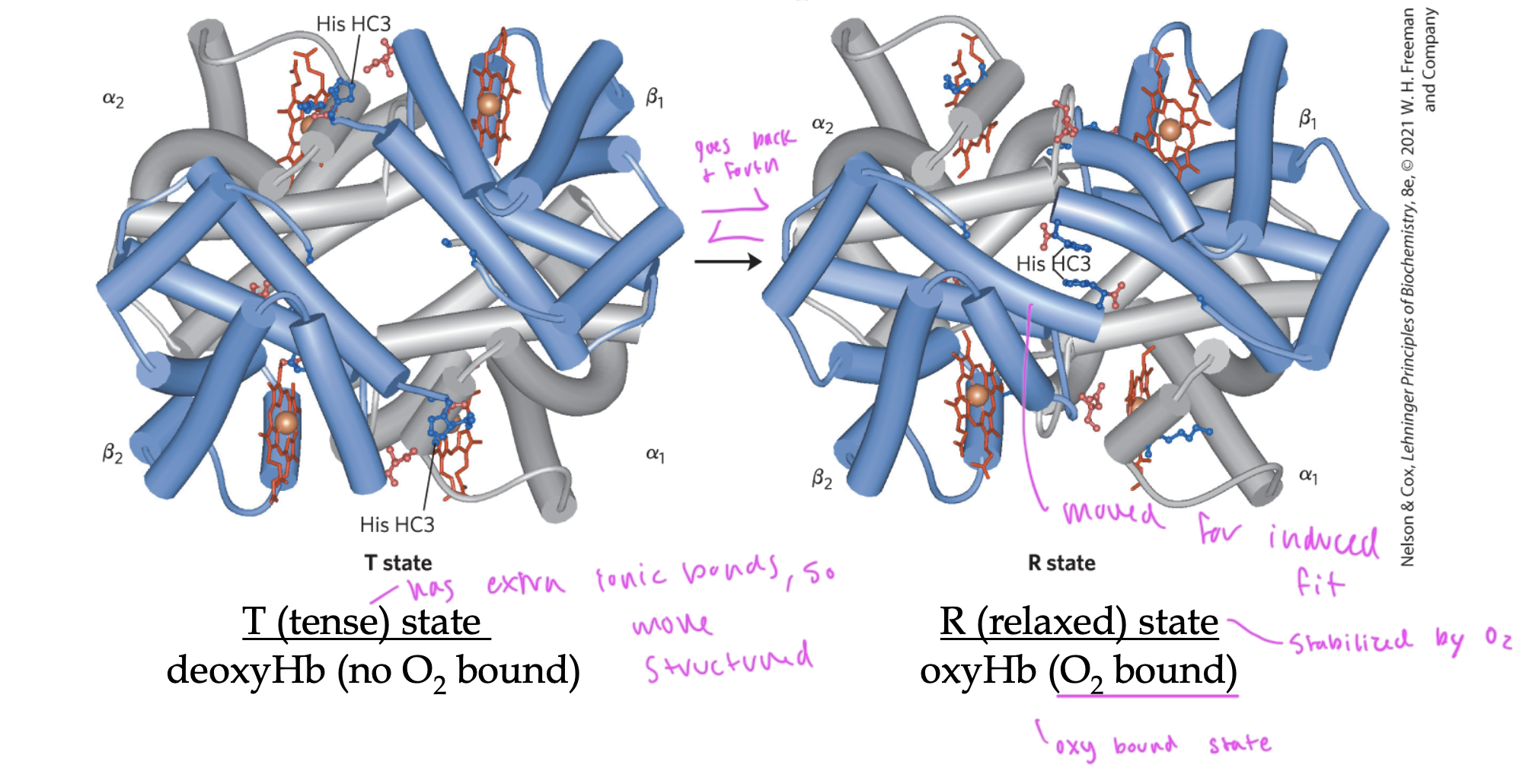
O2 binding to Hb triggers it to go from ____ state to ____ state
T to R state
so it can fit the O2 inside it
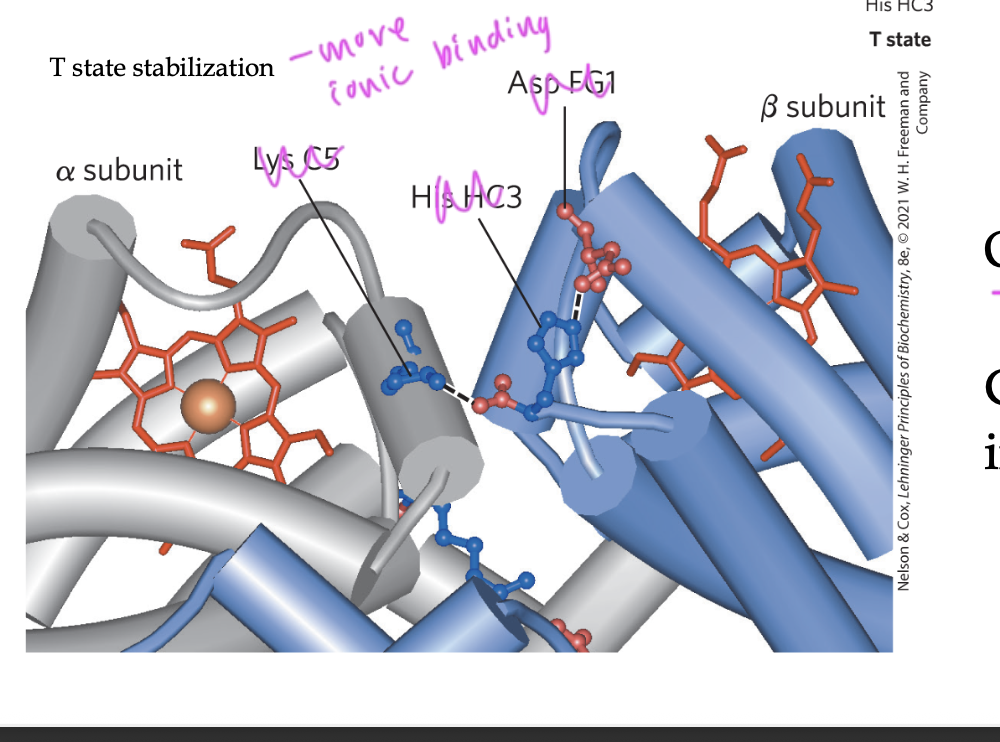
in order to do this, it must break those ionic bonds that kept Hb in T state
these ionic bonds were between the alpha1 and beta2 interface
the Heme group becomes more ____ when it is bound to oxygen in a Hb
planar
see how induced fit applies here
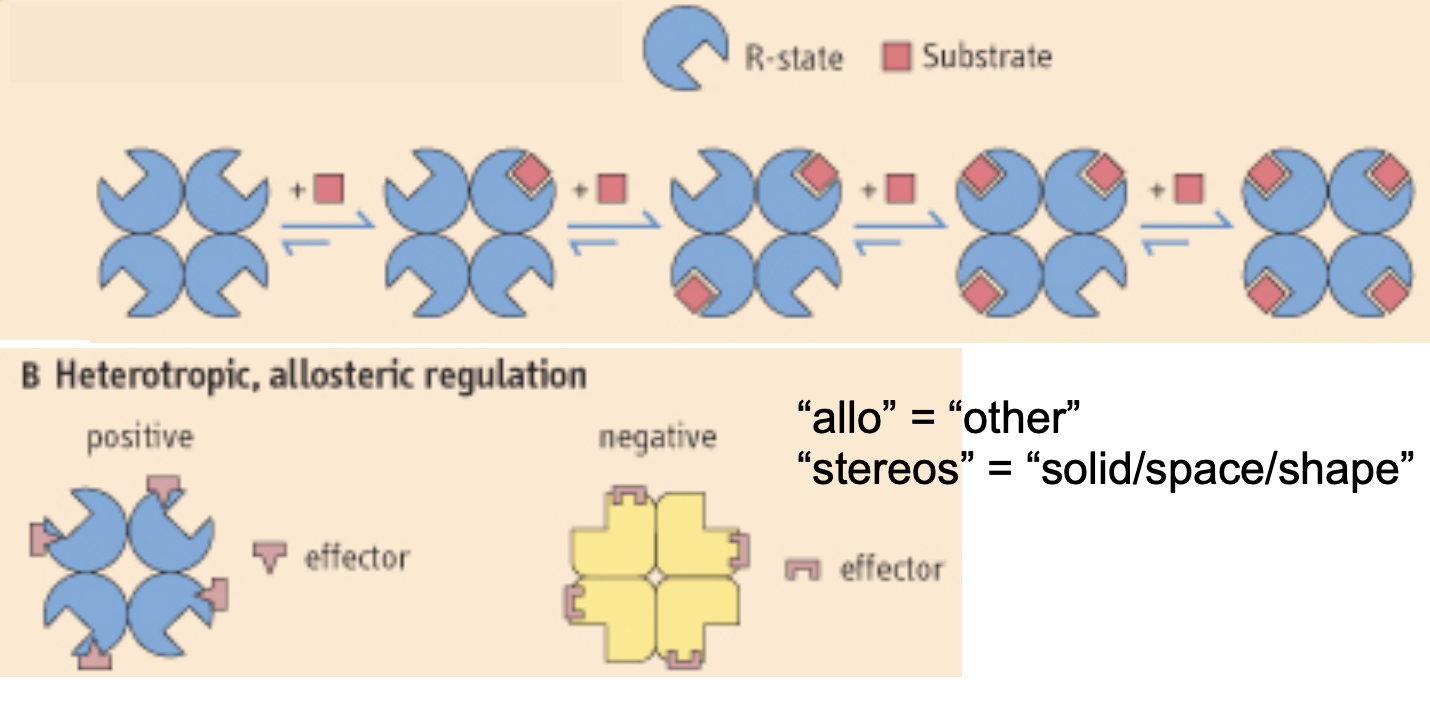
cooperative interactions
when the binding of one ligand affects the affinity of another ligand to bind on the same protein
this requires multiple subunits/domains on the protein
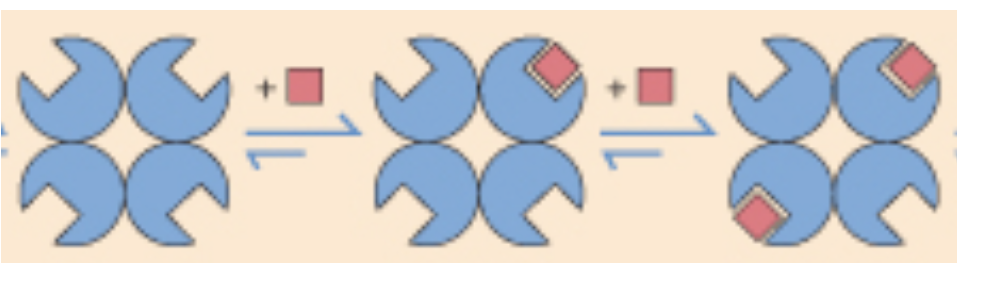
positive cooperativity
when the binding of a ligand incr the binding affinity of other ligands
negative cooperativity
when the binding of a ligand decr the binding affinity of other ligands
Hb subunits have ____ cooperativity when O2 binds to one subunit
positive
two models of cooperatively in ligand binding
concerted model: when all subunits must be in the same state, so once one subunit gets O2 and goes to R state, the rest go to R state and the binding of O2 to these following subunits is now favorable
sequential model: when one subunit changes state at a time, so when one subunit binds O2, it goes R and once ½ of the total subunits have O2 bound, it is now favorable for the rest to go R and gain O2
in each model, each subunit that changes makes it easier for the next subunit to change
scientists do not know which model is actually correct for Hb
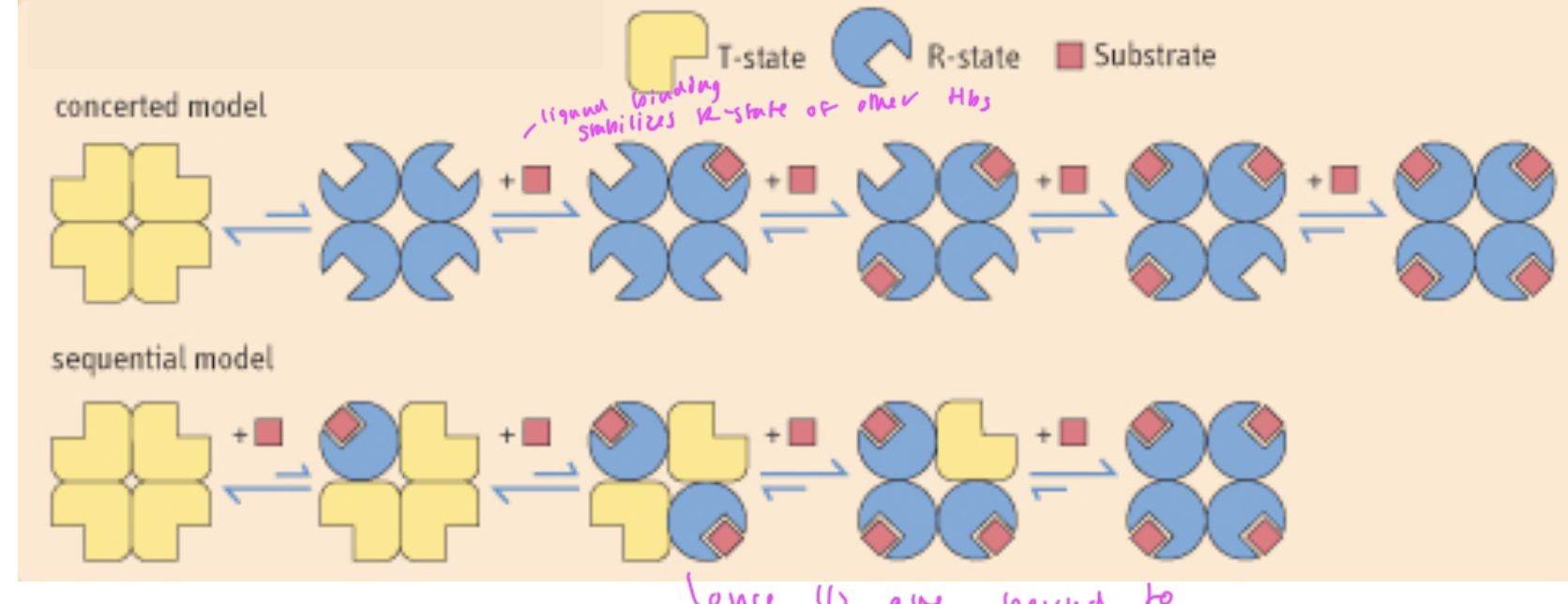
can Mb bind to O2 in a cooperative manner?
no
since the Hb structure has multiple binding sites, its binding curve is
sigmoidal
since it slowly transitions from low to high affinity for the O2
so Hb is highly sensitive to changes in oxygen levels
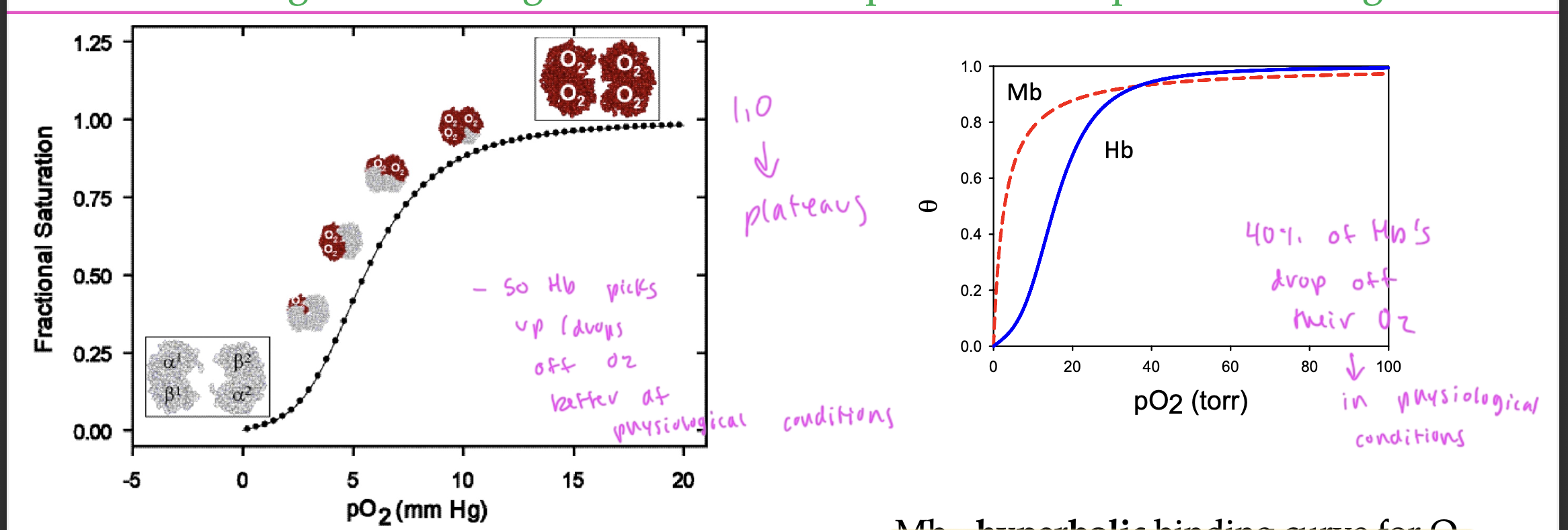
Hb picks up and drops off O2 pretty well at
physiological conditions
40% of Hb’s drop off their O2 in physiological conditions
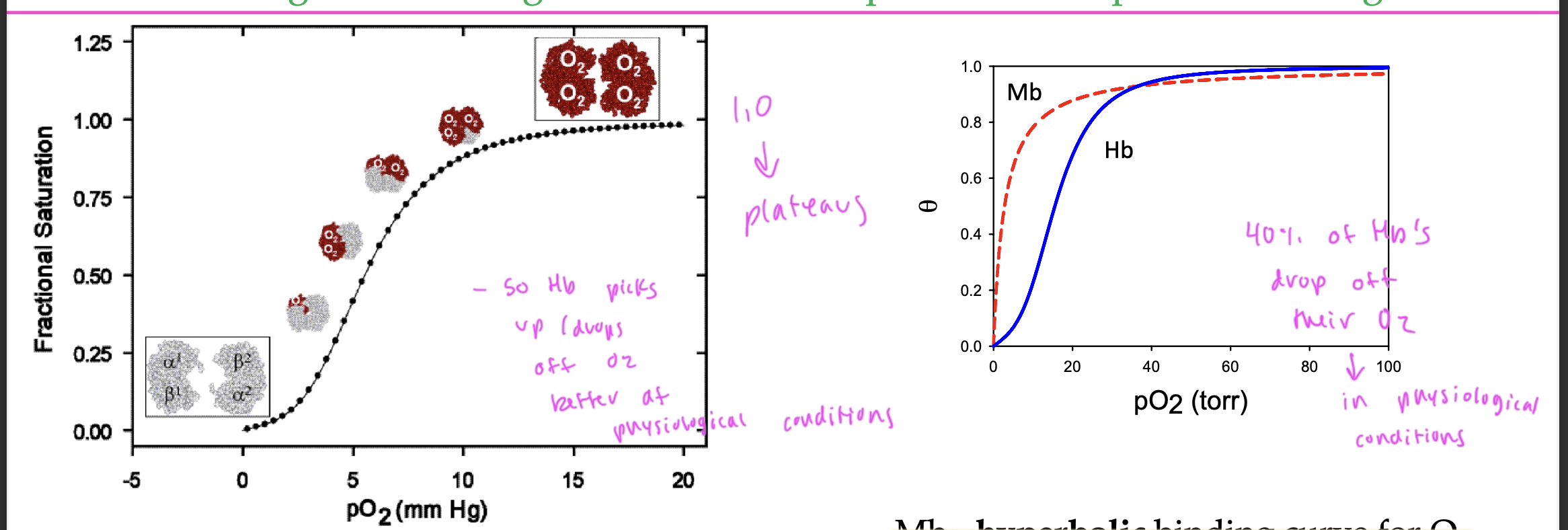
difference between Hb and Mb binding curves for O2
Mb—> hyperbolic
Hb—> sigmoidal
in order for something to effectively transport a ligand, it must
be able to drop off the ligand
so it must decr in affinity at some point to do this
ex: in certain tissues
see how Hb drops off O2 in tissues but holds onto it very tightly in the lungs
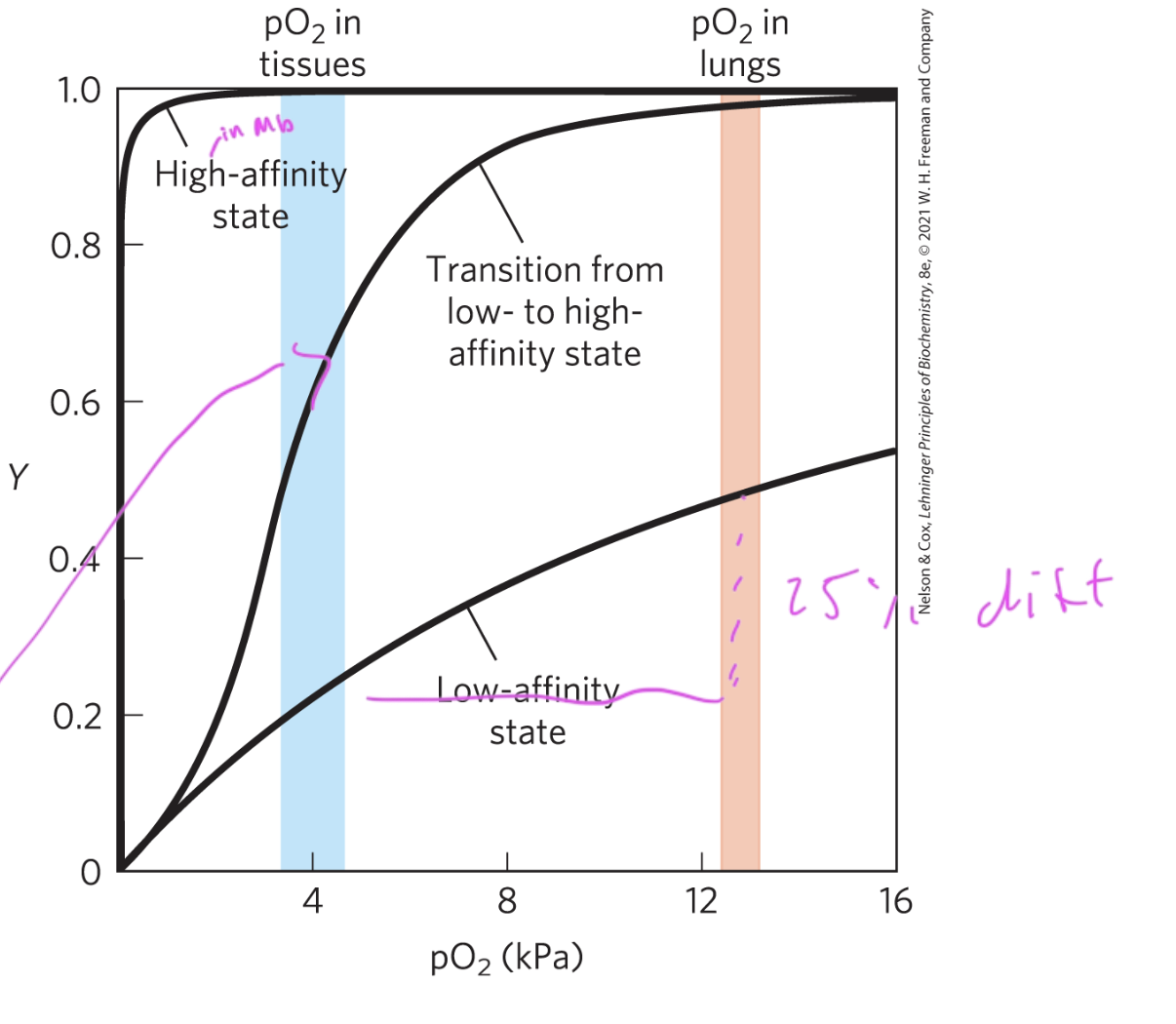
a ____ curve shows that cooperative binding its occurring
sigmoidal binding
for the regulation of ligand binding, _____ must be reversible
ligand binding
the binding of ligands other than oxygen affect the ______ of Hb
oxygen-binding properties
things like protons, CO, CO2, Cl-, and BPG affect the binding of O2 to Hb
they can shift the binding curve left or right
allosteric proteins
when the binding of one ligand affects the binding of another ligand to a different binding site on the same protein
positive allosteric proteins: when the binding of one ligand incr the affinity for other ligands
negative allosteric proteins: when the binding of one ligand decr the affinity of the protein for other ligands
____ allosteric regulators stabilize Hb in the T state
negative
is Mb a cooperative protein?
no
but Hb is
allosteric effectors
homotropic: when the binding of one type of ligand affects the binding of another of the same type of ligand
heterotropic: when a molecule different from the ligand affects how a ligand binds
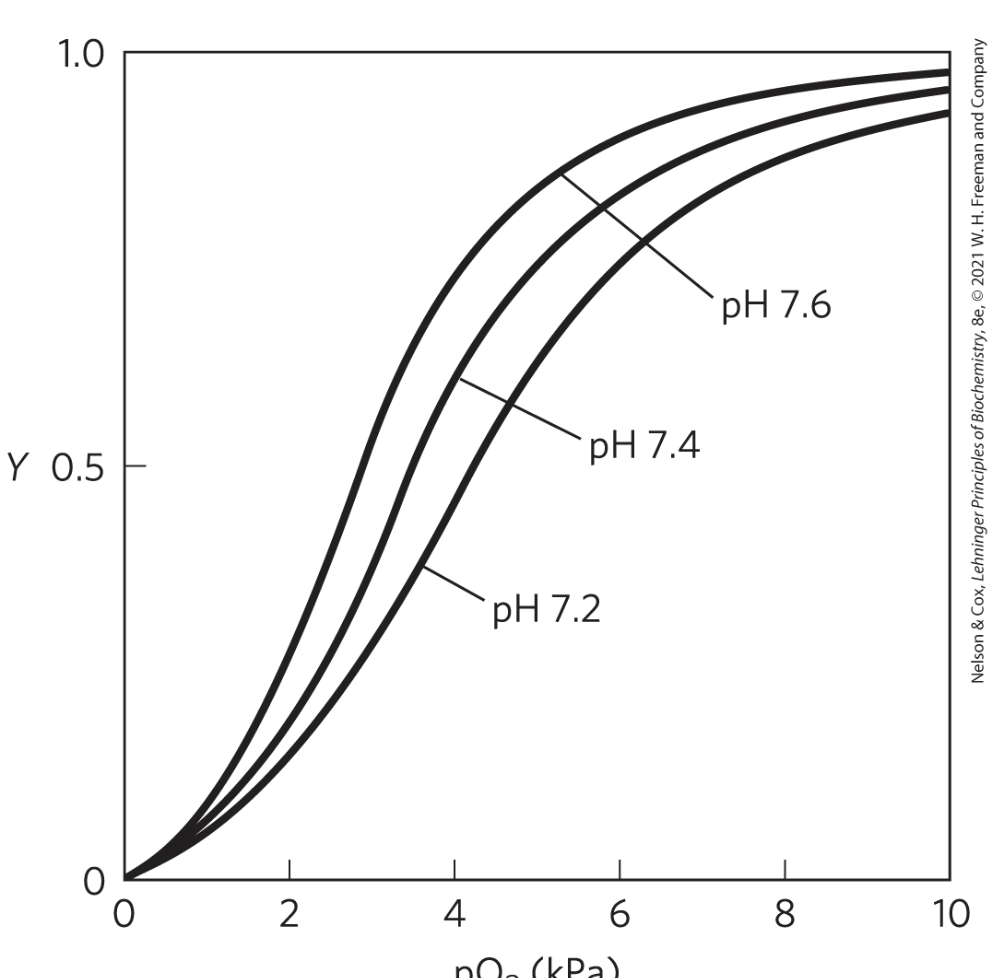
effects of pH on O2 binding to Hb
see how the binding of H+ to certain ionizable groups on Hb shifts it to T state
so H+ is an antagonist of O2 by binding in place of it on Hb
when pH decr, oxygen releases from Hb more easily
actively metabolizing tissues excrete acid, lowering the pH and promoting oxygen release from Hb
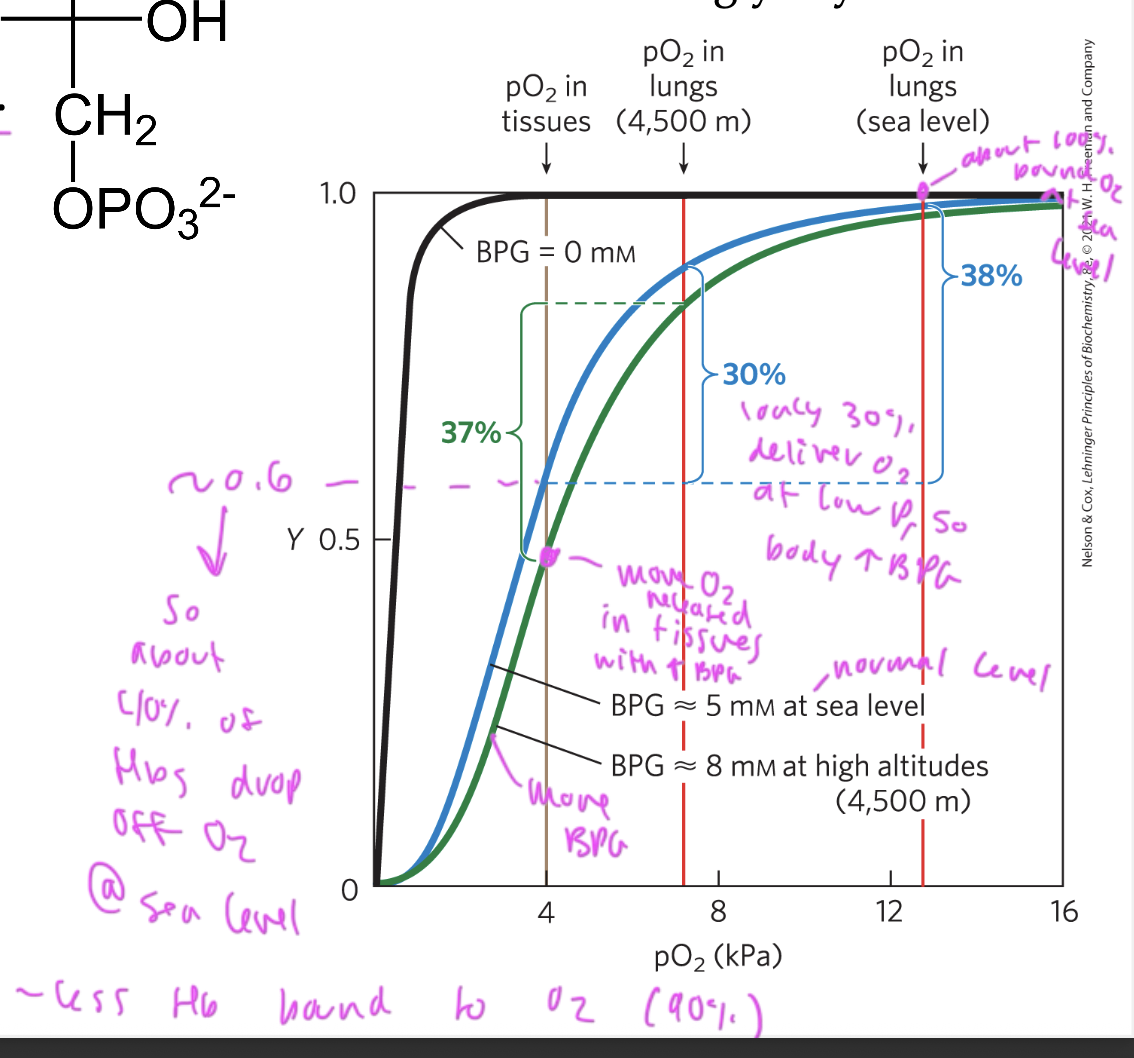
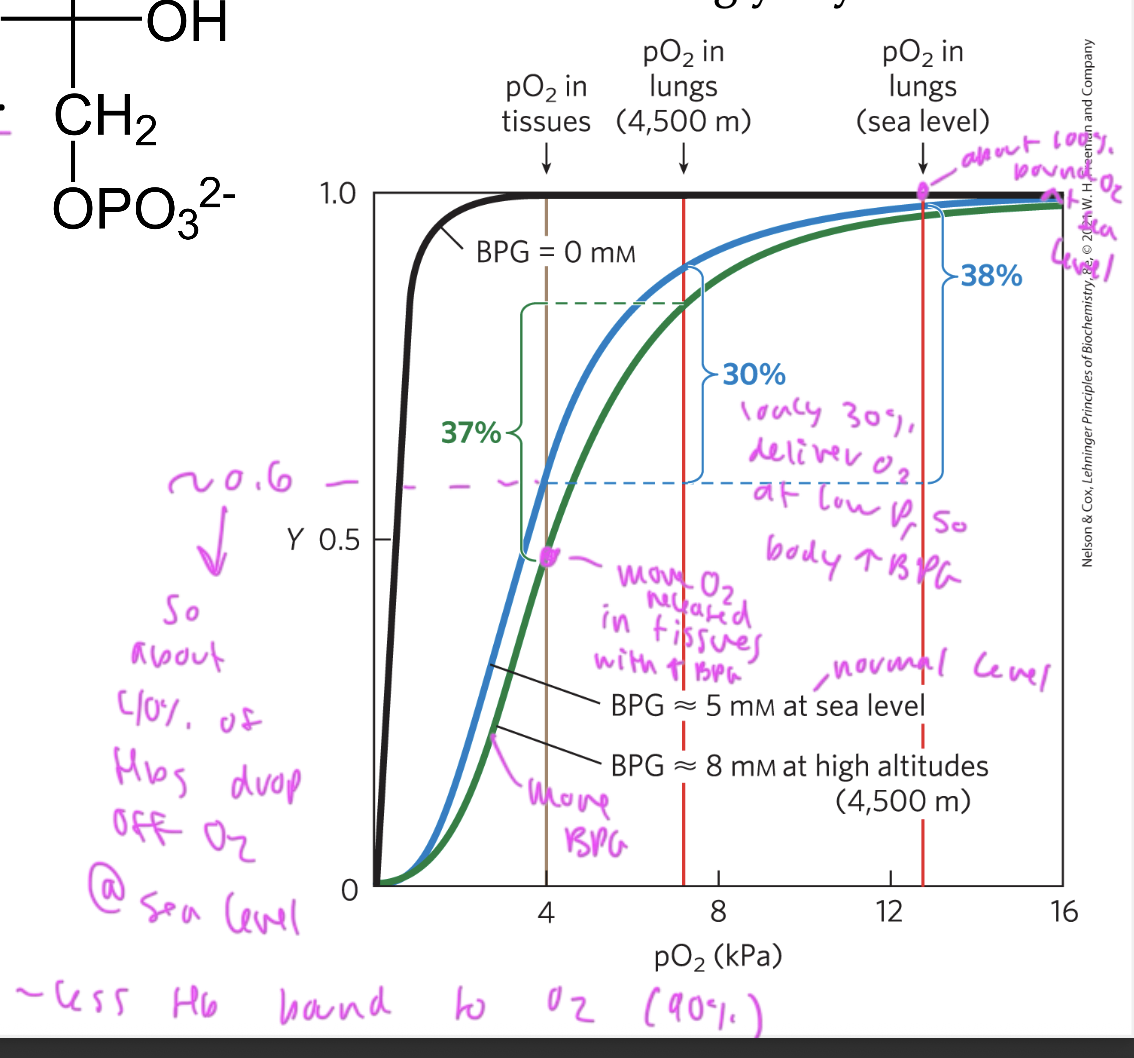
effect of BPG on O2 binding to oxygen
BGP binding to Hb promotes the release of oxygen (shifts curve right)
BPG is 2,3 biphosphoglycerate
is stabilizes the T state of Hb by crosslinking two beta subunits
when Hb is in the R state, the central cavity its too tight for BPG to bind, so oxygen binds
BPG shifts the oxygen saturation curve of Hb to the right so that more is released (it lowers the affinity of oxygen for Hb)
without BPG at low pressure: makes it so that the Hb start holding less O2 and therefore have less to deliver to the tissues, you want BPG to help get more delivered so it drops off more at tissues and shifts curve right
CO2 is a ____ effector of O2 binding to Hb
heterotropic
when CO2 is released, it can bind to Hb, preventing O2 from binding, so now more oxygen is released as the Hb is holding CO2
diff in graph with BPG vs without
with BPG, oxygen can be better delivered at high altitudes (low P) since at low pressure, less Hb is bound to oxygen (30% oxygen gets delivered at low P so the body incr BPG, causing more to be released)
at sea level (normal P) about 40% of oxygen gets released from oxygen from the lungs to the tissues
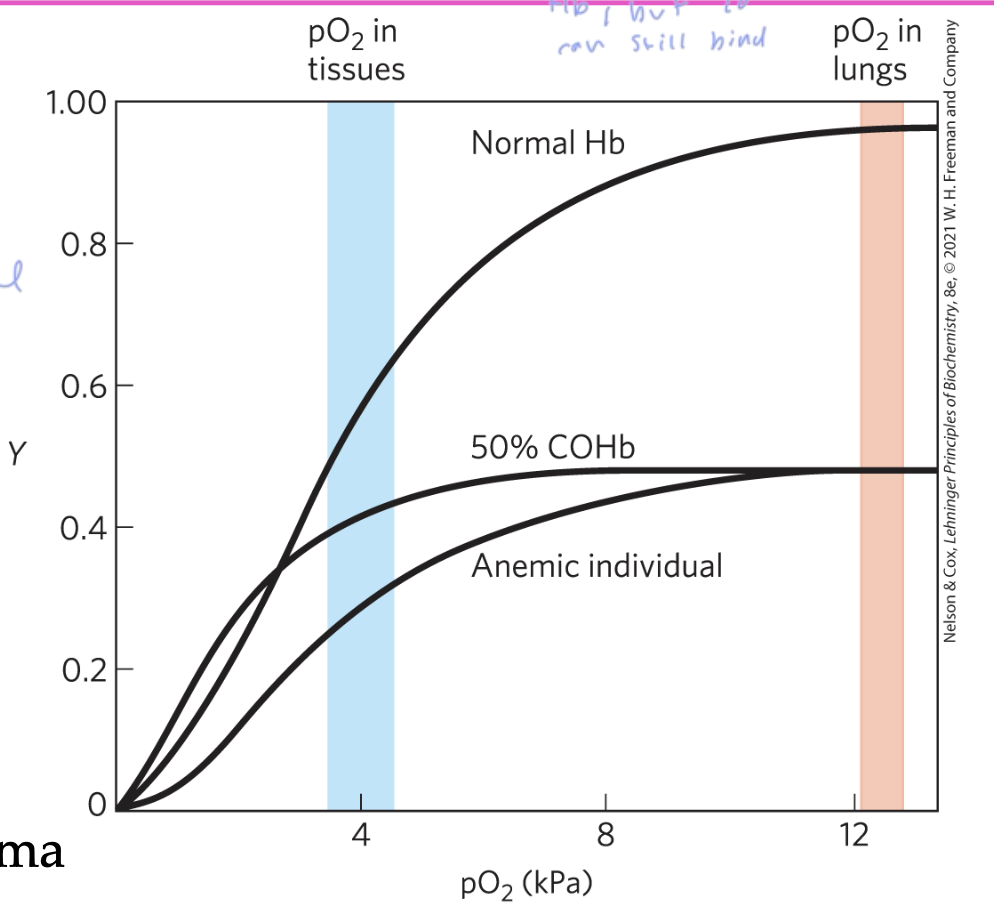
about ___% of Hb in tissues have O2 bound
60%
the more CO bound to Hb in your body from smoking, the less
the less Hb freed to bind O2 your body has
if you have about 10% of CO bound Hb, you would have a few symptoms
20-30% causes severe headache and 60% causes death
smokers and people with lung, heart, or a blood disease are at a greater risk for anemia
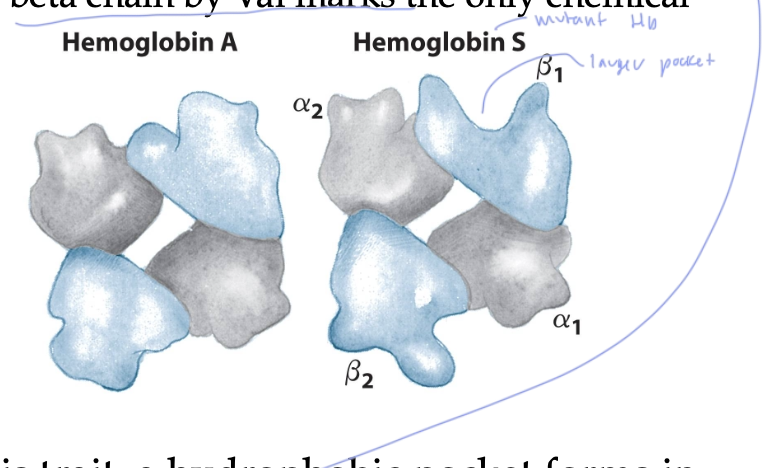
what at the genetic level causes sickle-cell anemia?
when the Glu residue at position 6 in the beta-chain is replaced by a Val
turns Hb A into Hb S, which is a mutant
this Hb S has a larger beta- 1 pocket on the Hb, which allows for the polymerization of many Hb molecules, which causes many lattices to form when the Val side chain of one deoxy Hb S interacts with the next deoxy Hb S
the Hb S don’t hold oxygen as well
these lattices form all sorts of shapes that cause RBCs to sickle, and they struggle to move through the blood stream, making you feel very sick
happens when someone with sickle cell anemia has an episode, and only becomes a problem when someone with the mutation is in a stressful situation
Hb S is less _____ than Hb A
soluble in RBCs
sickled cells are more
elongated, causing buildup/ hard to move the RBCs through the bloodstream
it takes a while for new ____ to build back up after an episode for someone with sickle cell anemia
RBCs
the sickled cells eventually get degraded, but it can takes weeks for new ones to replenish
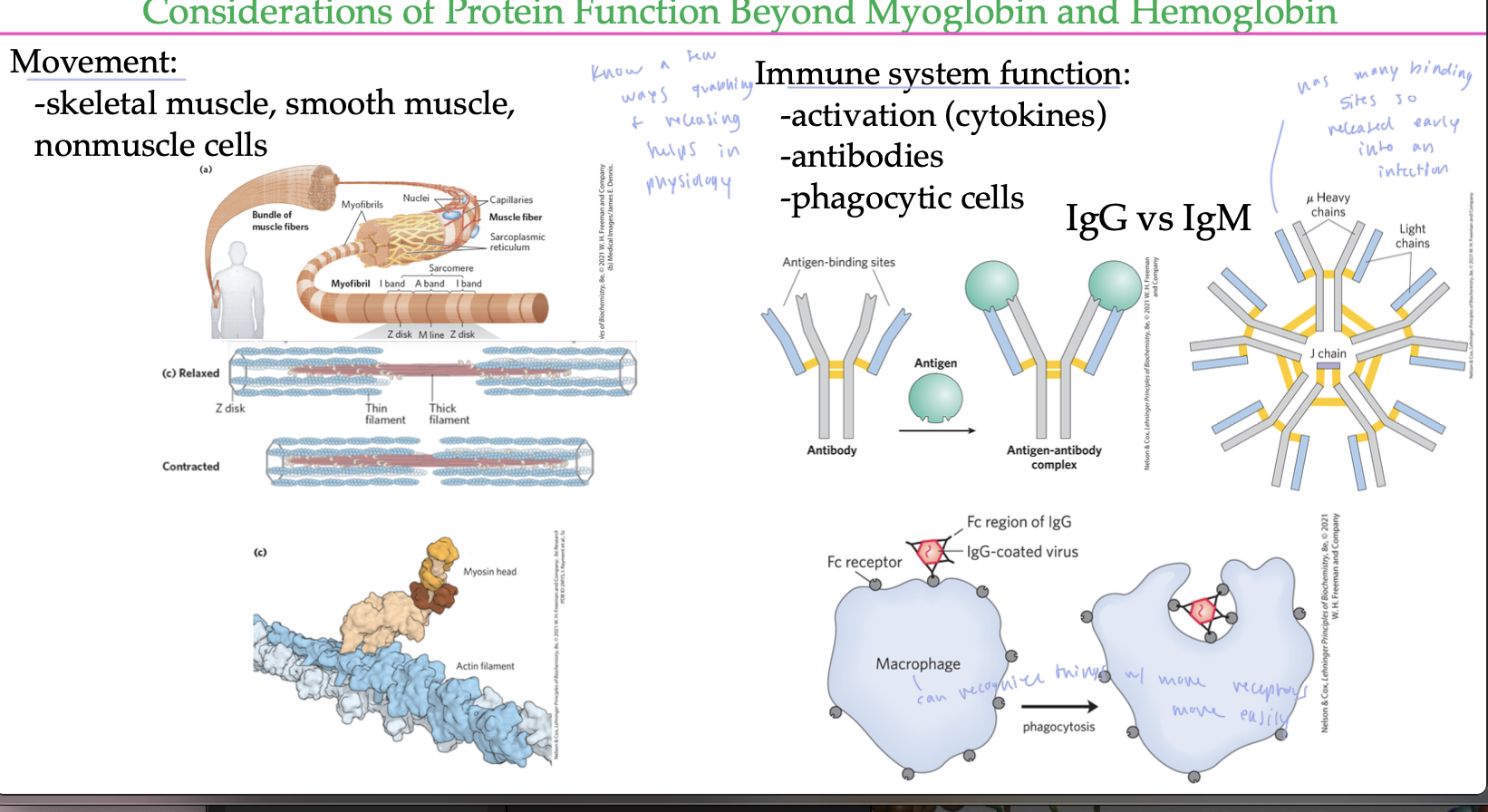
ways grabbing/releasing things help in physiology
movement: skeletal muscle, smooth muscle, nonmuscle cells
in the immune system, it is used for antibodies to form and release from things, also allows macrophages to recognize things to consume