Cell Communication
1/30
Earn XP
Description and Tags
Lecture 11
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
What are the 4 modes of signalling and please describe them also??
Endocrine (secretion of ligands via bloodstream and the ligand secreted is called a hormone which is produced in endocrine cells)
Paracrine (ligands diffuse directly through the extracellular matrix and act as local mediators and this can be seen in immune responses)
Neuronal (can generate quick electric impulses which are then converted to chemical signals and this is done via neurotransmitters)
Contact dependant (direct contact of cell between ligand and receptor which are embedded in each cell).
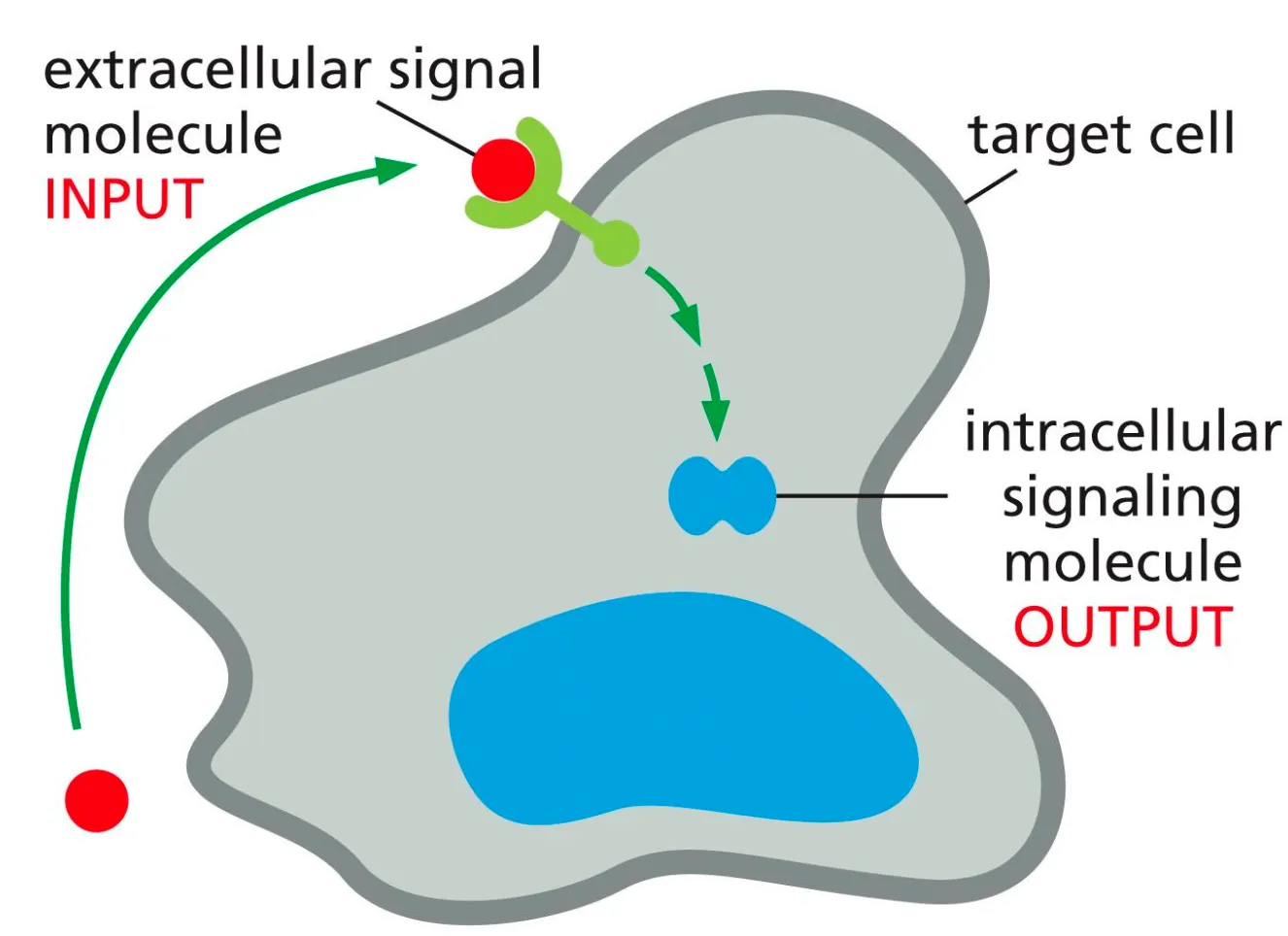
What is the general mechanism of Signal Molecules??
Target cells have proteins which are receptors and can recognise ligands. Signal transduction happens when the receptor on the target cell receives an incoming extracellular signal and then produces intracellular signalling molecules, which alter the cell behaviour.
The response of a cell to a certain signal depends on whether that cell has the given receptor for the ligand. Each receptor is only activated by a single ligand.
The reaction mechanism of a cell on the signal depends on which cascade of intracellular signals the cell-surface receptor can generate and how that effects the effector proteins.
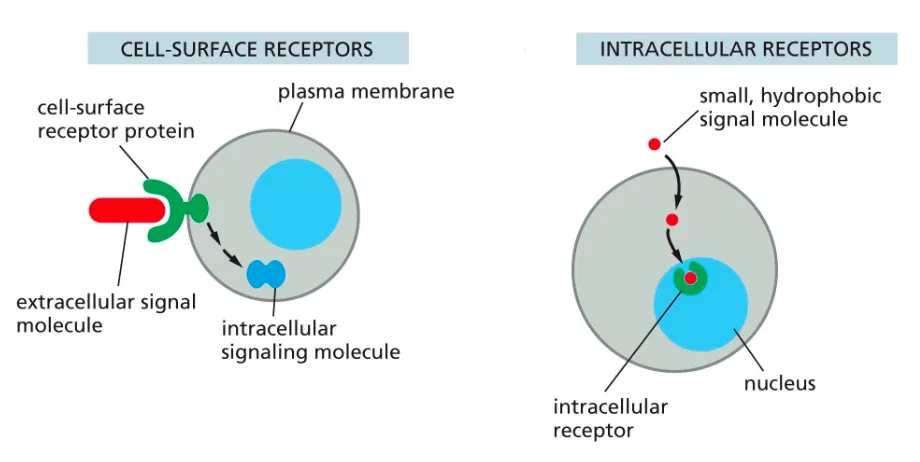
Define Receptors?? and what are the two types.
Receptors can be cell surface ones or intracellular receptors.
The intracellular receptors, are found in the cytosol of the cell and recognise hydrophobic and smaller molecules which are able to pass through the membrane.
Cell surface receptors help large and hydrophillic molecules get targeted and recognised in order for the intracellular events to take place.
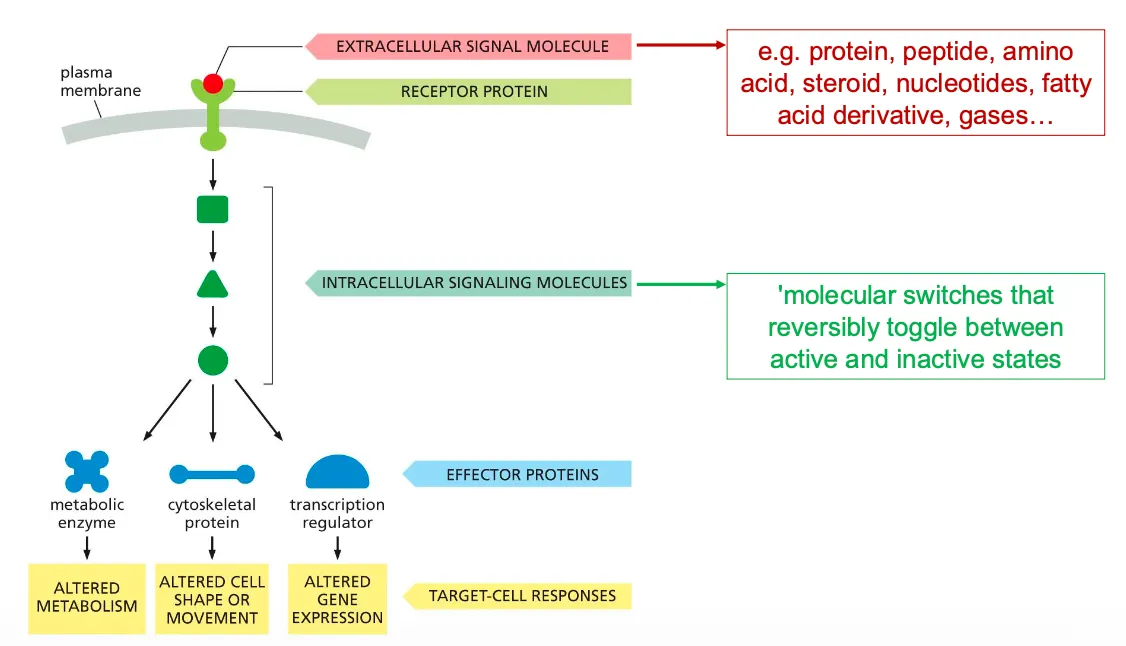
What happens when ligand and receptor bind??
After a transmembrane receptor and ligand bind a cascade of events takes place, where intracellular molecules pass on signals until they reach effector proteins which eventually causes the target cell to respond.
The image beside shows the components of the intracellular signalling pathways.
These signalling processes can have certain functions such as relaying the signal and spreading it out in the cell, they can also amplify the signals received, they can also detect the signal and integrate the signals, they should also be able to distribute the signal to more than one effector proteins and they can also modulate the response to give feedback to the processes.
Amplification of Signals and the role of scaffold proteins??
These signalling processes can have certain functions such as relaying the signal and spreading it out in the cell, they can also amplify the signals received, they can also detect the signal and integrate the signals, they should also be able to distribute the signal to more than one effector proteins and they can also modulate the response to give feedback to the processes.
Scaffolds are structural proteins that can bind to receptors and recruit the signalling proteins. Can also bring together different kinds of proteins. They are also part of the intracellular reaction and help in all these functions.
(Then the process broadens out, amplification takes place. Smaller intracellular molecules are small and can spread out.
Then there are effector again which can cause the cellular response.)
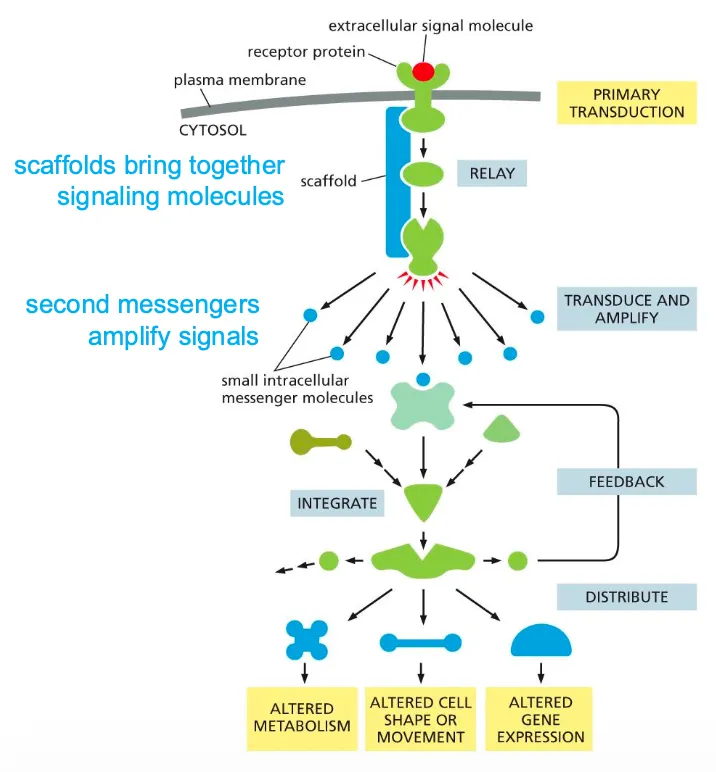
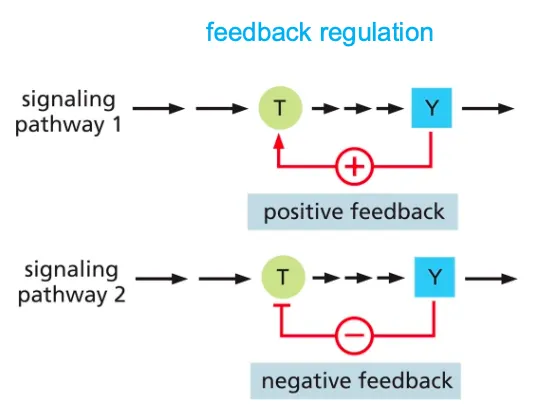
Explain the mechanism of feedback regulation???
Feedback Regulation is a means of control in a cell and it can either boost or weaken the response of a signal in a cell.
Positive feedback will enhance the response to initiate a signal and negative feedback will inhibit the earlier component to avoid further response.
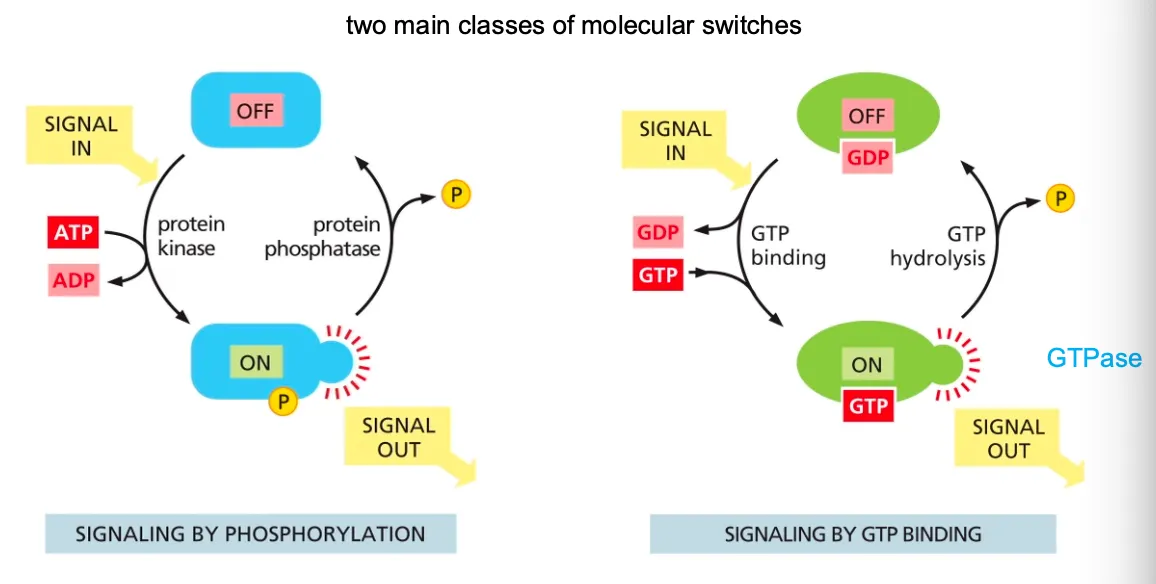
How do proteins act as switches??
We also have proteins that act as switches and the mechanism works as the following:
Mostly these switches consist of activation or inactivation through phosphorylation. In these cases the protein kinase attaches a phosphate group to the switch protein and in the opposite direction the protein phosphatase will remove that phosphate when inactivating the switch.
Many of these phosphorylation proteins are organised in cascades, where the one protein kinase phosphorylates another and so on. Common examples of such events are serine/threonine kinase or tyrosine kinase.
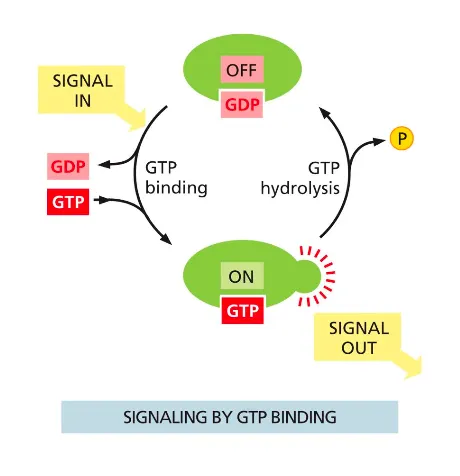
GTP binding will cause the molecule active (GTPases are inactive with GDP and when bound to GTP they are active). The GTPase controls its own hydrolysis, in which it then also deactivates when the bound GTP becomes a GDP.
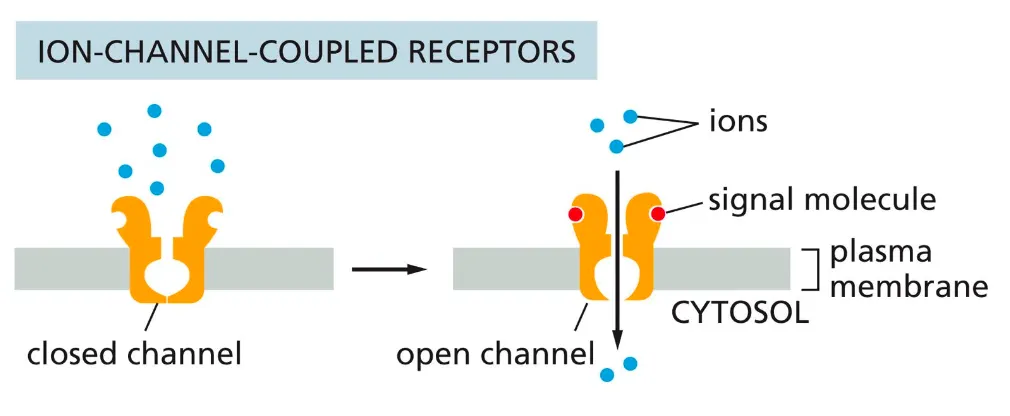
Ion-channel coupled receptors
Ion-channel coupled receptors
(ligand-gated ion channels, often neurotransmitter receptors) - regulate membrane permeability for certain ions by altering the membrane potential and creating an electrical current.
The ion channel coupled receptors are responsible for the rapid transmission of signals across synapses in the nervous system. They transduce a signal in form of neurotransmitters which causes the membrane potential to alter.
This binding of the neurotransmitter to the receptor causes a conformational change in the receptor causing it to open and allowing the flow of ions in the membrane. Based on their electrochemical gradients, the ions flow in or out the membrane.
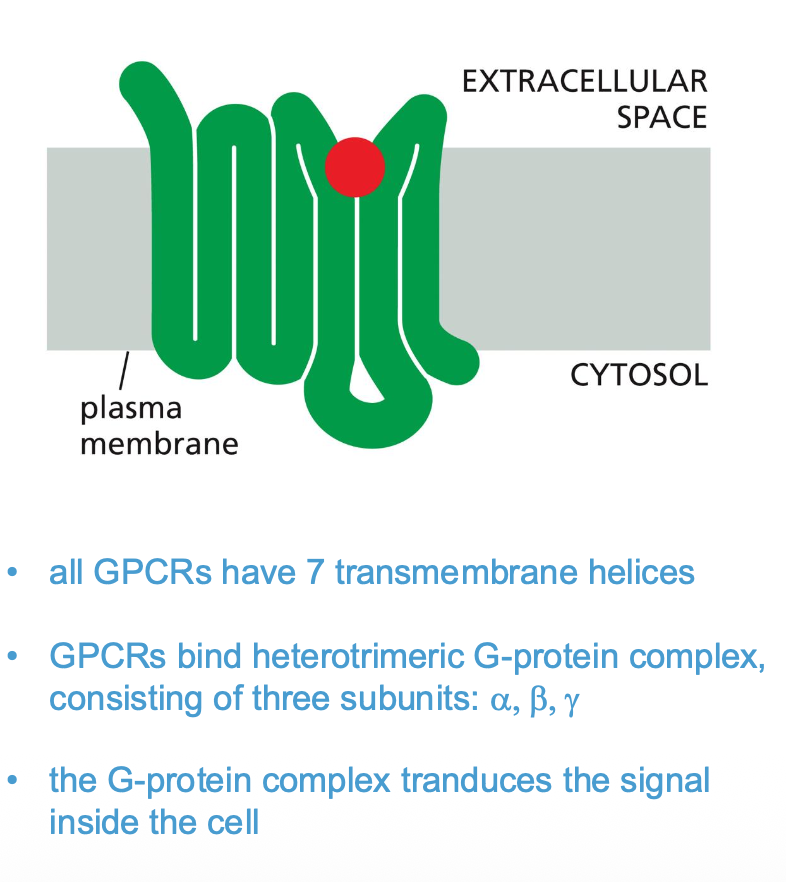
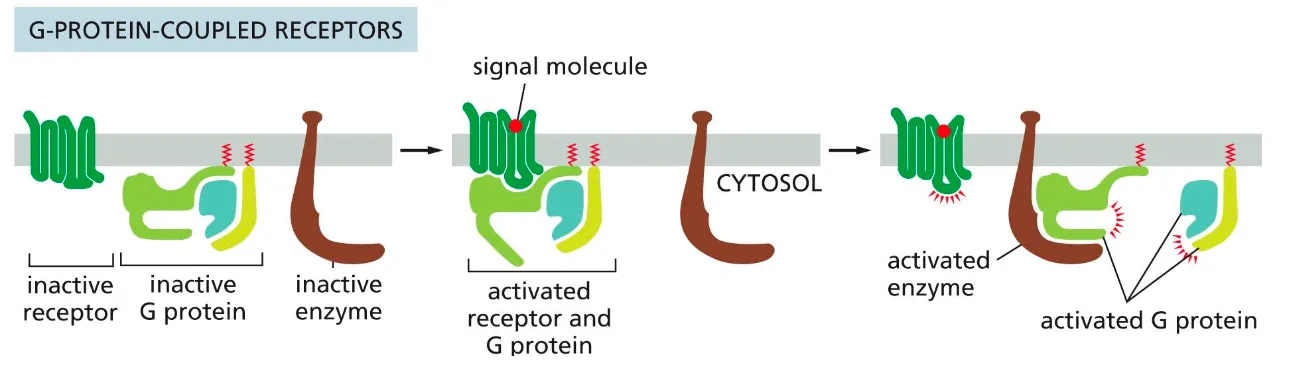
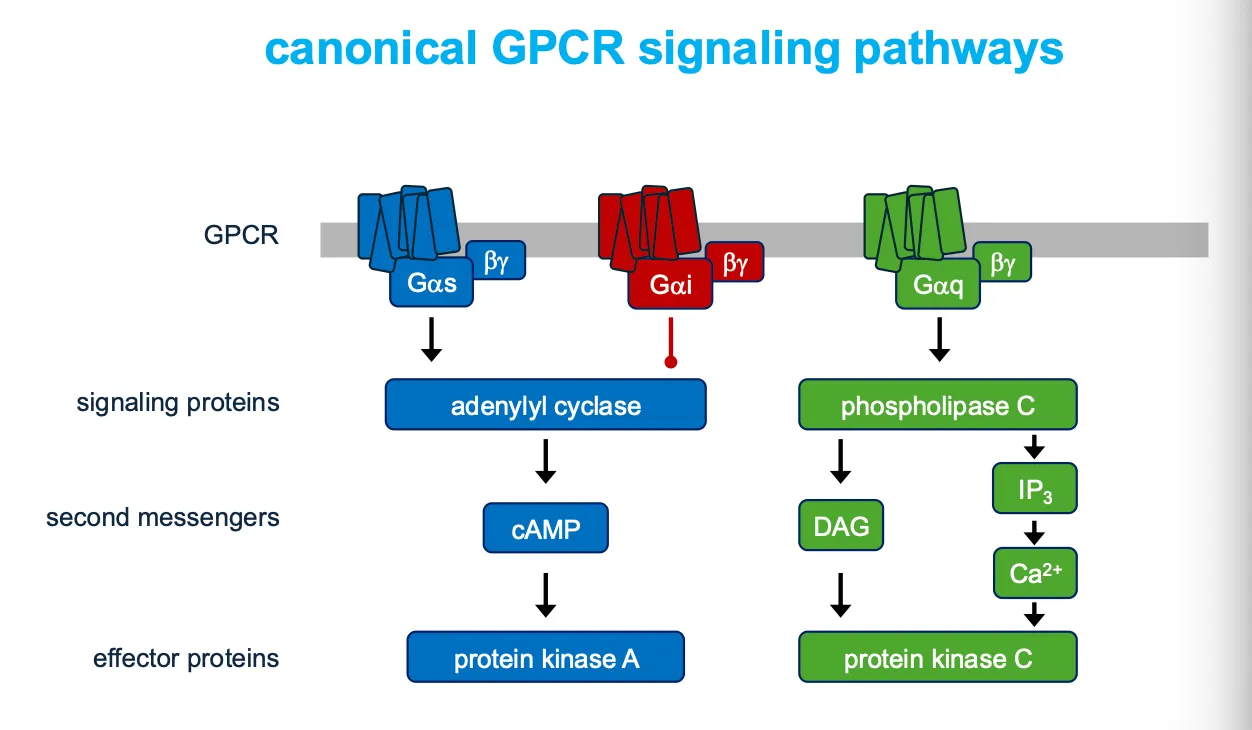
G-protein coupled receptors (GPCRs)
These receptors activate membrane-bound GTP binding proteins, which then activate or inhibit an enzyme or ion channel in the membrane, which initiates the cascade of events of intracellular signals).
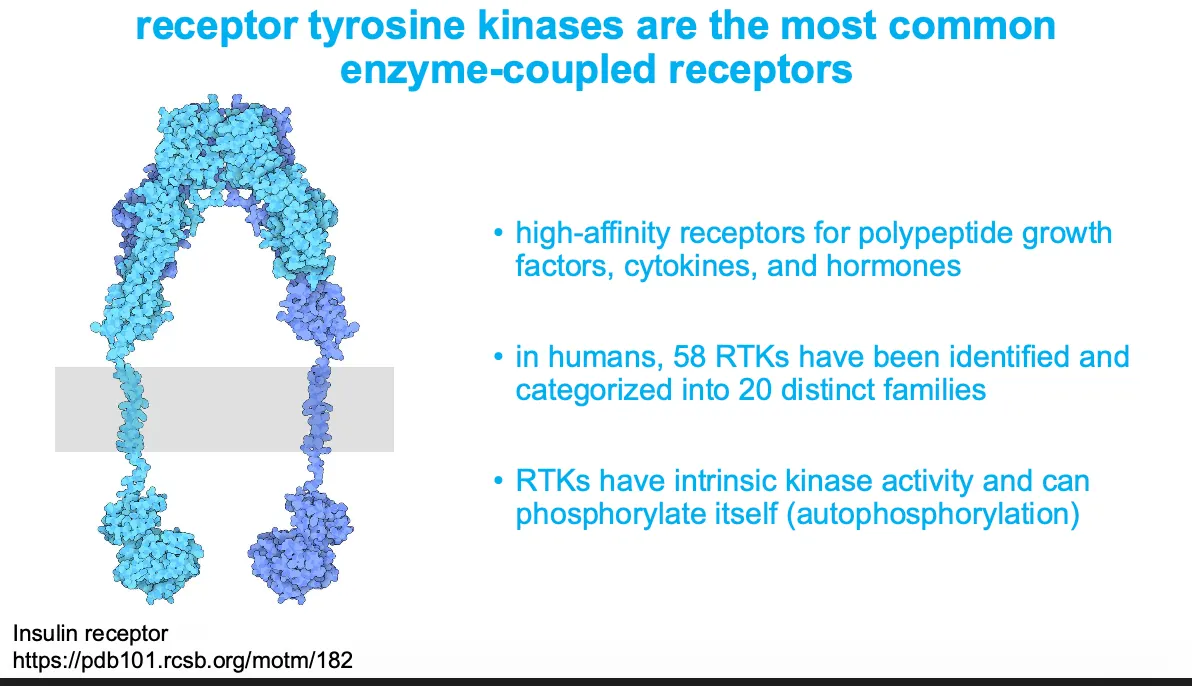
Enzyme-coupled receptors
(Receptor tyrosine kinases) - these receptors either act as enzymes or associate with enzymes inside the cell and when they are stimulated then they can activate many intracellular pathways.
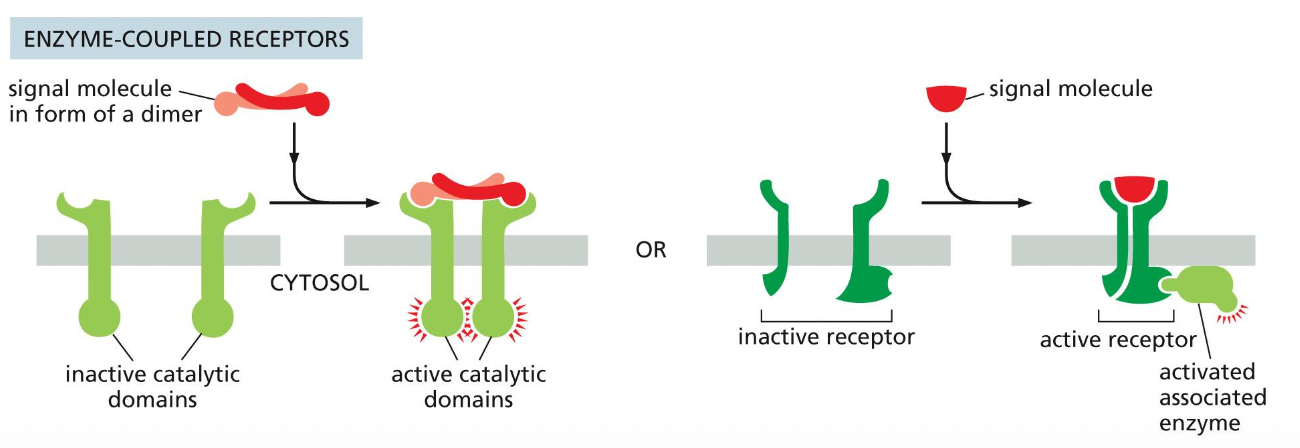
They are in subunits but when they become active then they become a dimer and that makes them begin signalling. Sometimes the molecule needs to be activated itself then activated the cell.
How are GPCRs Activated???
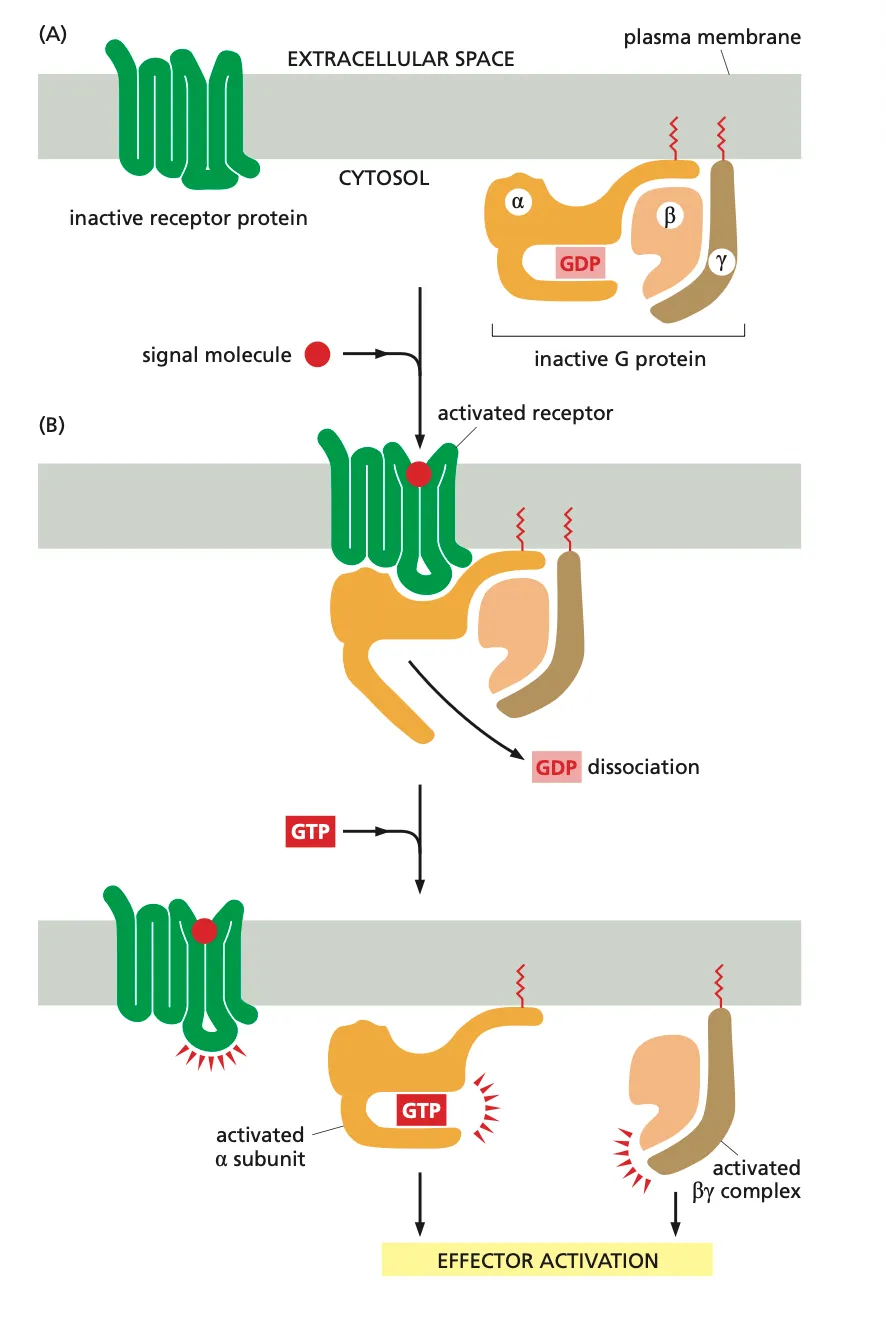
Firstly we have a signal molecule (ligand) which binds to the receptor and undergoes a conformational change and that causes the receptor to bind to a G-protein. (Lipidated molecules attached to the amino acids which makes sure they are bound to the membrane before they can be received by a receptor.)
In the G-protein this movement activates a conformational change in the G^alpha subunit which causes the dissociation of GDP from the G-protein.
This GDP molecule is rapidly exchanged with a GTP molecule. Now this induces a conformational change of the G-protein complex and the hetrodimeric G-protein complex dissociates into Galpha and Gbeta(gamma).
Both these complexes then activate various downstream effector proteins.
What is the G-alpha unit??
G alpha subunit are molecular timers because they have intrinsic GTPase activity
The active, GTP-bound G-alpha subunit hydrolyses the GTP to GDP
In this way the G-alpha subunit inactivates itself with a certain delay
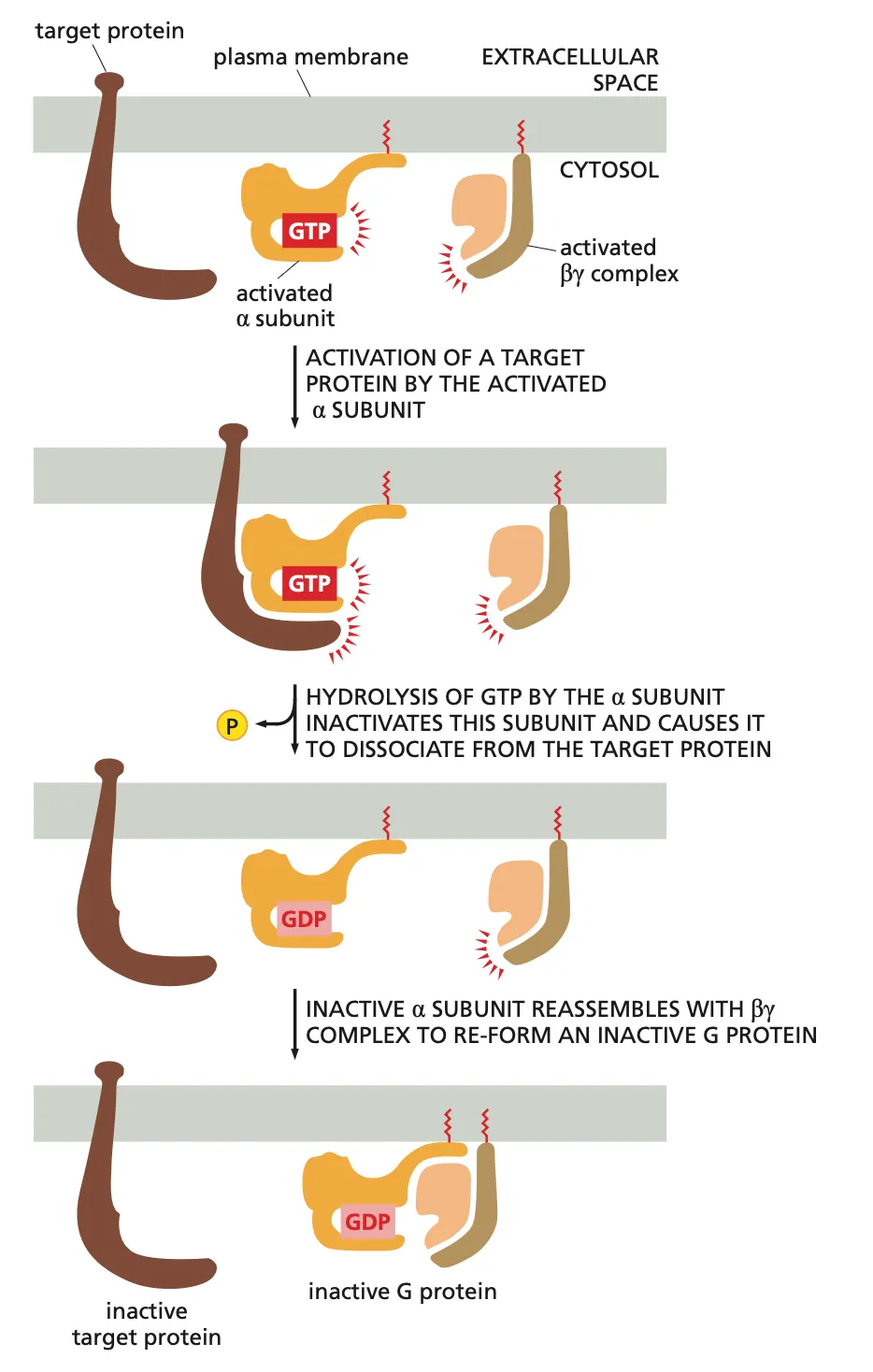
What happens if a ligand activates the alpha unit of the G-protein??
Basically what happens is after the Ligand binds to the G protein coupled receptor the G protein Alpha unit is activated with the binding of GTO and it binds to the target protein activating the target protein.
Then the alpha subunit hydrolyses the GTP into GDP which inactivates the subunit and causes it to dissociate from the target protein.
This inactive alpha subunit now ressembles back with the beta subunit to form the inactive complete G protein and now it can wait for a new signal molecule (ligand).
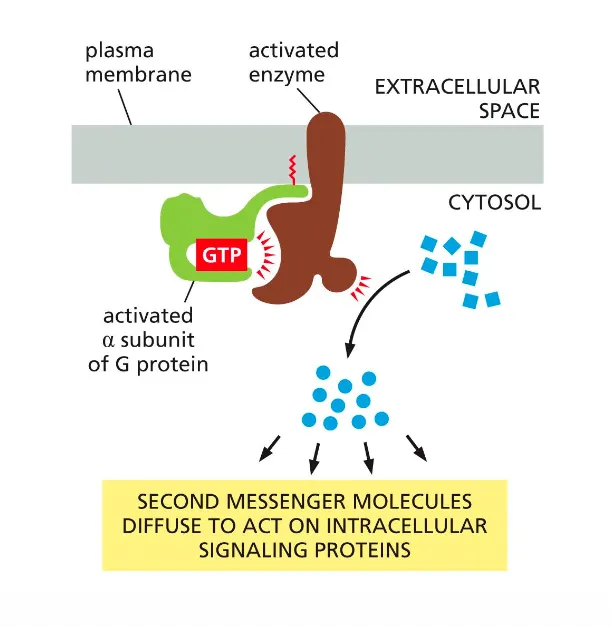
How are G-protein signals amplified?
The production of diffusible second messengers amplifies G-protein signalling.
Each activated enzyme releases these secondary messages which amplify the signal because they can further bind to more receptors.
What do the different subunits of the G-proteins do??
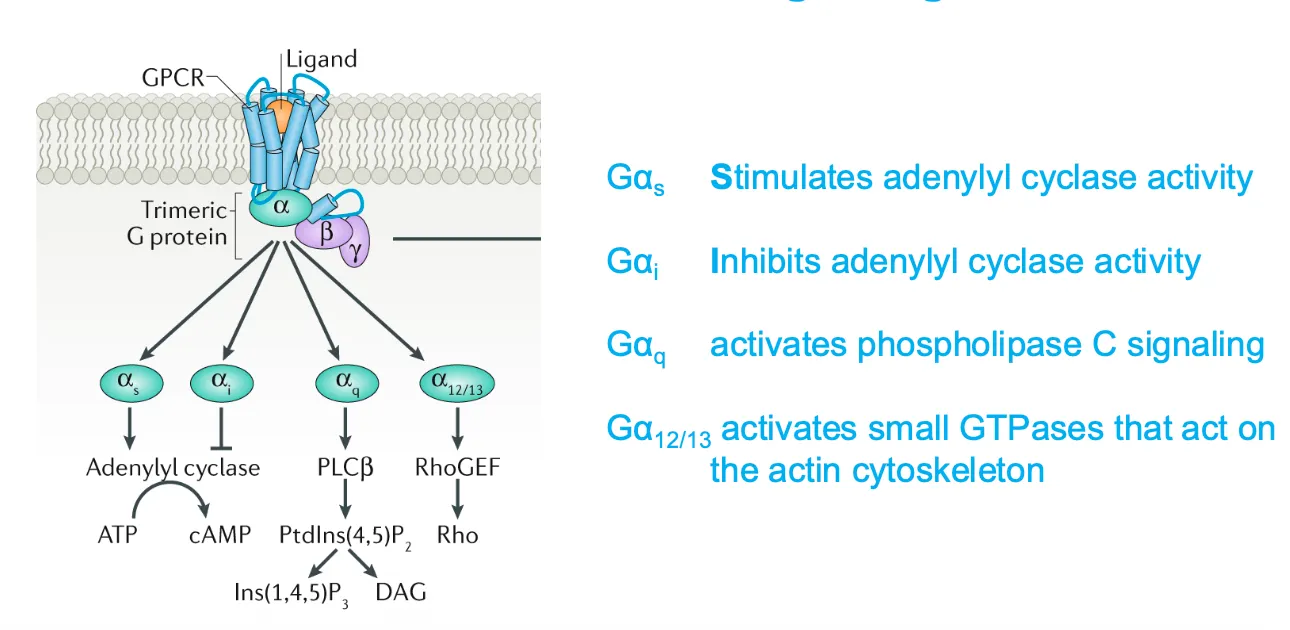
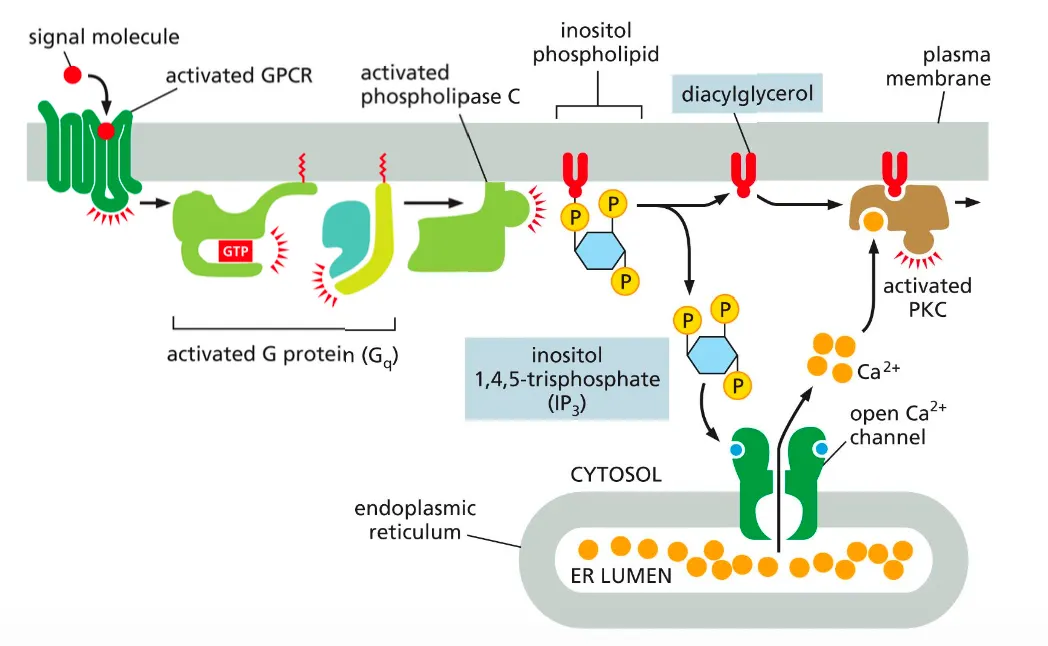
How does the G-alpha-Q activate the PLC pathway?
Phospholipase C activates two signalling pathways. Which will increase the intracellular concentration of Ca ions.
This is done on the following way:
After a GPCR activates a G protein Gq another membrane bound enzyme called phospholipase C is activated and this phospholipase cleaves molecule called inositol phospholipid.
This cleavage produces two messenger molecules called inositol 1,4,5 triphosphatase and diacylglycerol.
The IP3 is released into the cytosol, where it binds to Ca ion channels in the ER membrane and allows the flow of calcium ions into the cytoplasm of the cell. This Ca2+ in turn signals to other proteins, as we discuss shortly.
Diacylglycerol remains embedded in the membrane and helps activate protein kinase C (PKC). Once activated, PKC phosphorylates a set of intracellular proteins that varies depending on the cell type.
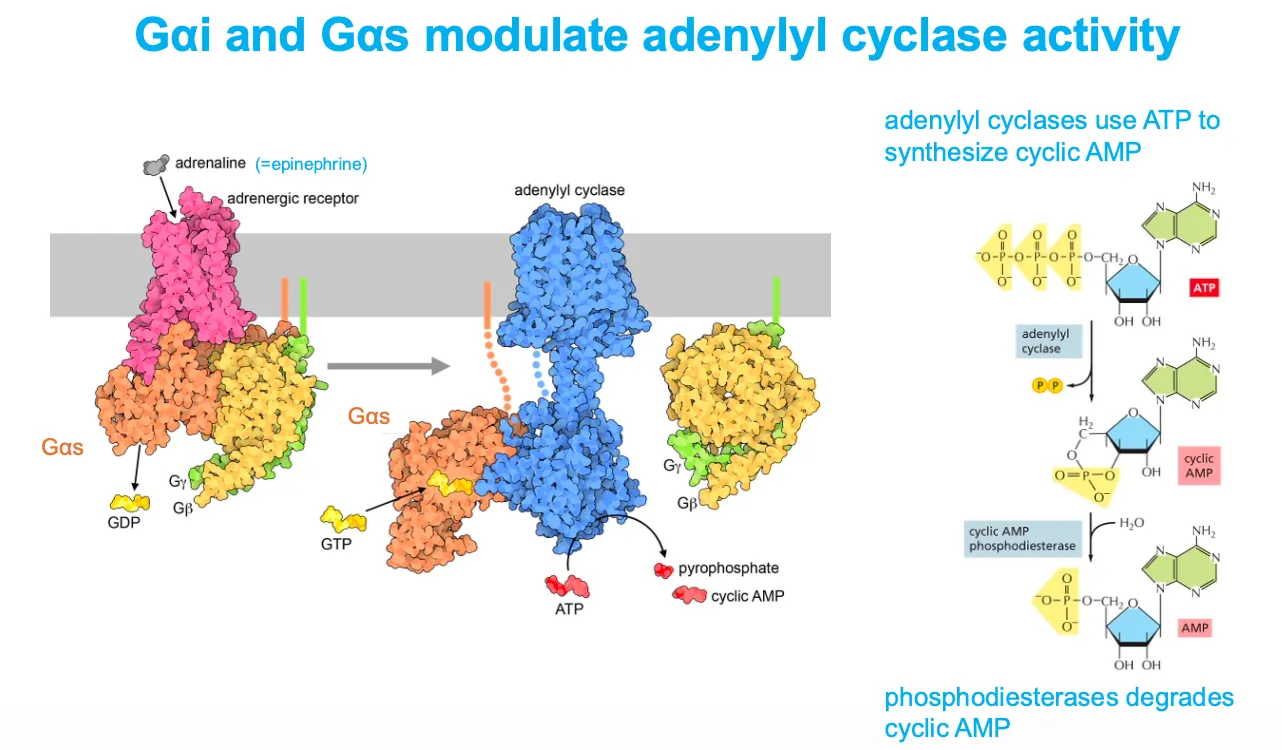
How do Galphai and Galphas modulate adenylyl cyclase activity???
When adrenaline binds a β‑adrenergic receptor, Gαs exchanges GDP for GTP, dissociates, and activates adenylyl cyclase, which converts ATP into cyclic AMP (cAMP); cAMP is later broken down to AMP by phosphodiesterases.
Conversely, Gαi (not drawn in detail here) inhibits adenylyl cyclase, lowering cAMP, so cells can fine‑tune cAMP signaling by balancing Gαs and Gαi activities.
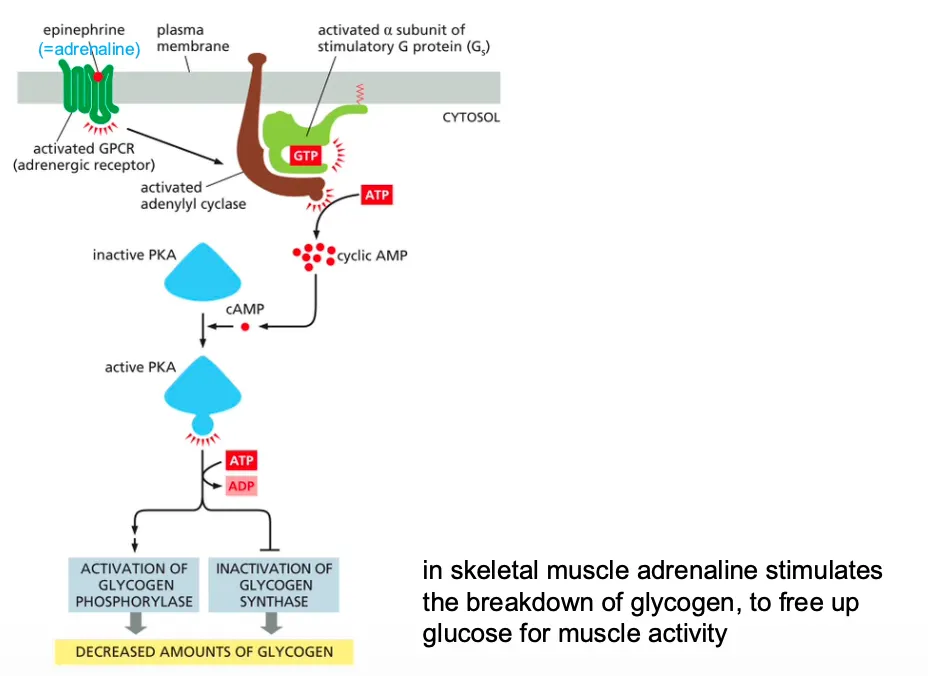
How does cAMP stimulates the activity of protein kinase A (PKA)??(adrenaline)
Epinephrine stimulates glycogen breakdown in skeletal muscle cells.
The hormone activates a GPCR, which turns on a G protein (Gs) that activates adenylyl cyclase to boost the production of cyclic AMP. The increase in cyclic AMP activates PKA, which phosphorylates and activates an enzyme called phosphorylase kinase.
This kinase activates glycogen phosphorylase, the enzyme that breaks down glycogen (see Figure 13–22). Because these reactions do not involve changes in gene transcription or new protein synthesis, they occur rapidly.
In muscle u get cAMP activation which in skeletal muscle adrenaline stimulates the breakdown of glycogen, to free up glucose for muscle activity.
→ In skeletal muscle adrenaline stimulates the breakdown of glycogen, to free up glucose for muscle activity.
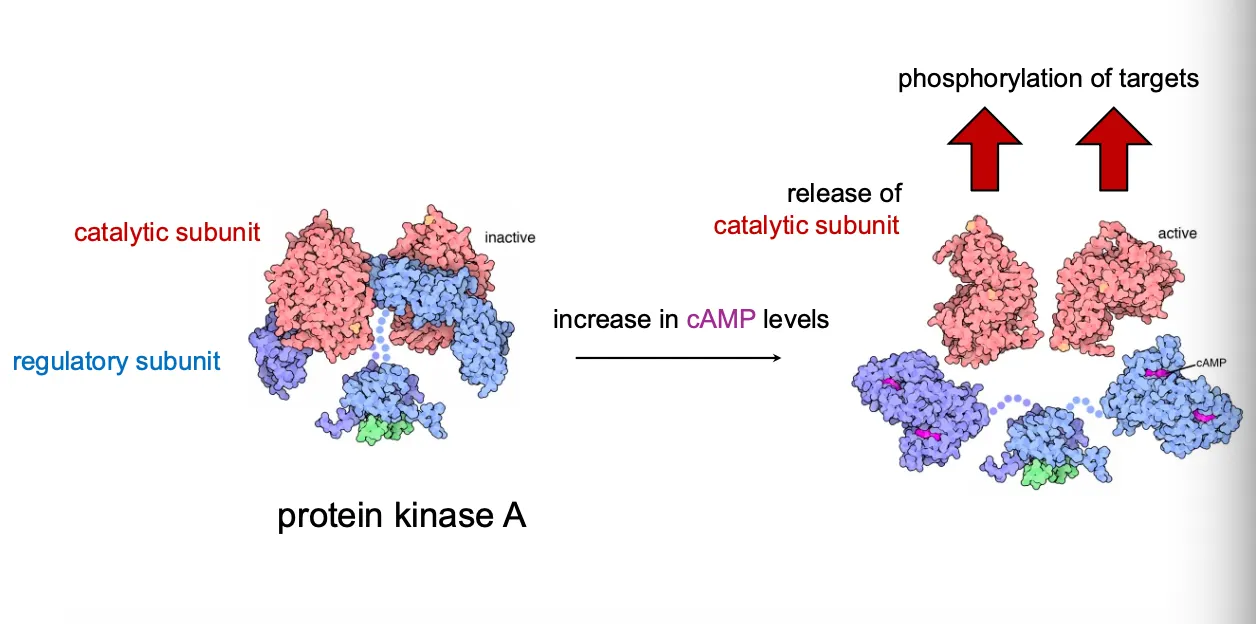
Kinase activation by release of catalytic subunits.
In the inactive PKA holoenzyme, two regulatory subunits bind and inhibit two catalytic subunits.
When cAMP levels rise, cAMP binds the regulatory subunits, causing them to dissociate and freeing the catalytic subunits, which then phosphorylate many target proteins.
Many kinases work like this and that is how they sense the change from secondary messengers
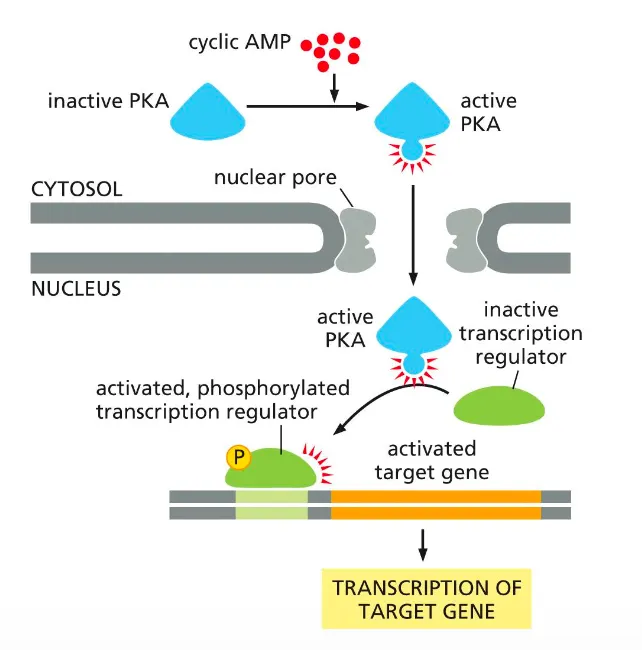
How does active PKA drive gene transcription???
cAMP‑activated PKA can regulate gene expression by phosphorylating transcription factors in the nucleus.
cAMP converts inactive cytosolic PKA into active PKA, which then enters the nucleus.
Active PKA phosphorylates an inactive transcription regulator, turning it on so it binds DNA and drives transcription of its target gene.
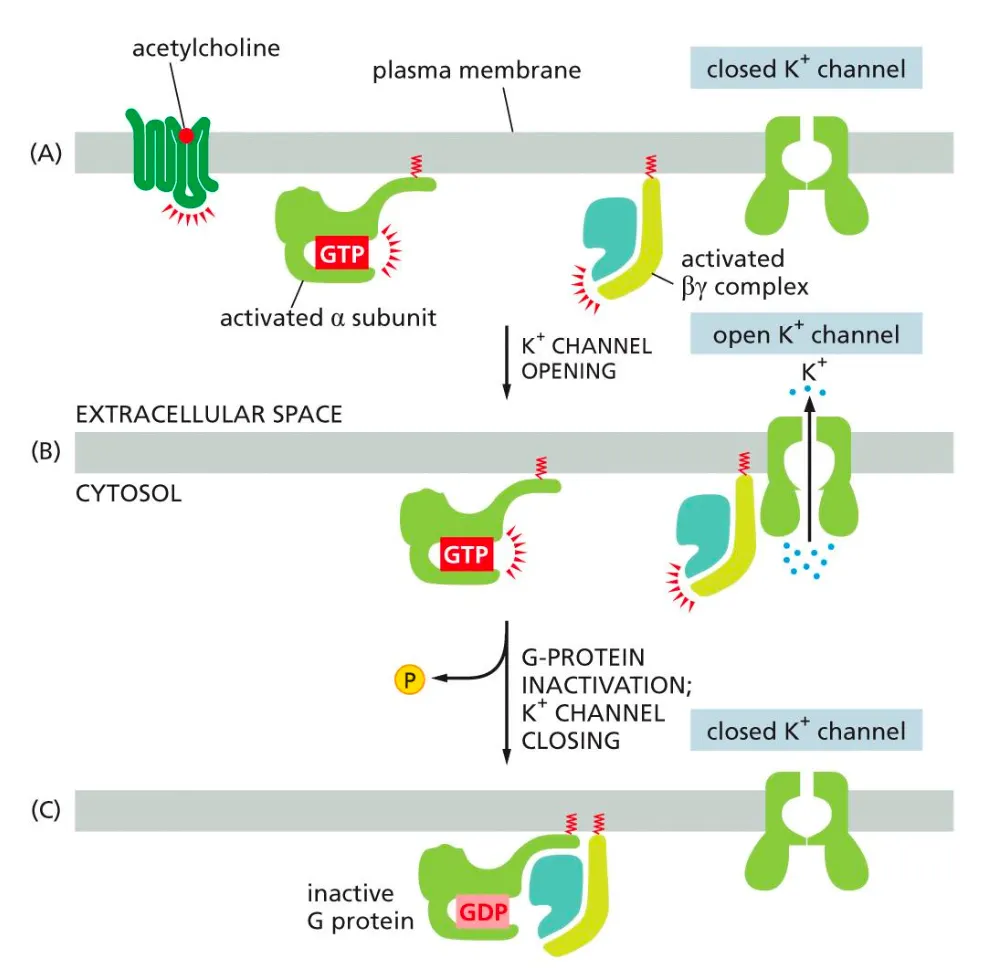
The activated G-beta complex can activate ion channels in the membrane. How??
Alongside the alpha subunit we also have the beta one, this can activate ion channels in the following manner:
When acetylcholine binds to the GPCR it activates the G-protein (both alpha and beta units). When the activated beta unit binds to the receptor it causes the receptor to open and K ions to flow out. The G-protein is then inactivated after binding to GDP, therefor the channel closes again
Overview of the CPCR Pathways
ENZYME-COUPLED RECEPTORS
How are they activated??
Receptor tyrosine kinases (RTKs) are activated by ligand‑induced dimerization and autophosphorylation.
A signal molecule (often a dimer) brings two RTK monomers together, forming a dimer that activates their cytosolic tyrosine kinase domains, which then cross‑phosphorylate tyrosines on each other’s tails.
The phosphotyrosines act as docking sites for intracellular signaling proteins, which bind, become activated, and launch downstream signaling pathways.
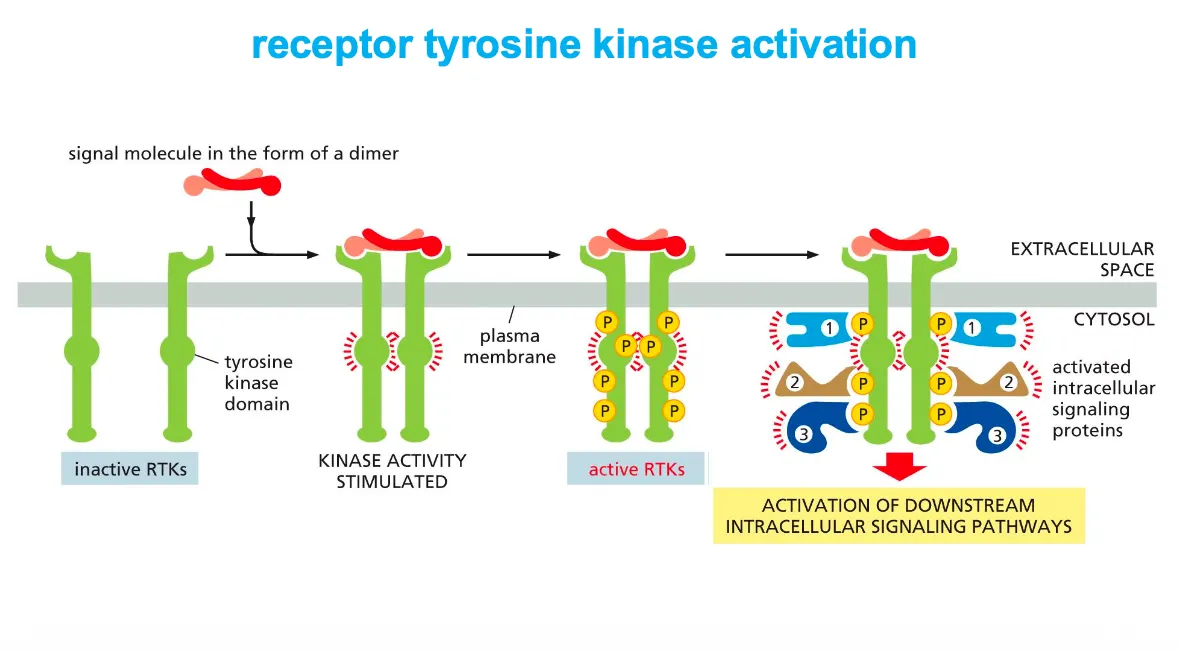
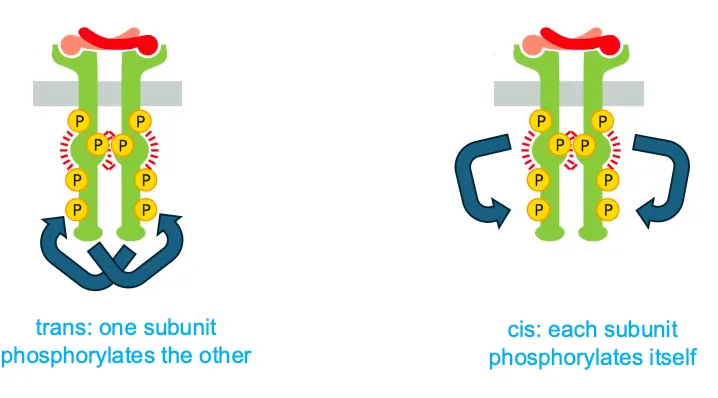
What is the difference between cis and trans activation??
Cis activation is when each subunit phosphorylates itself and trans is when they phosphorylate each other.
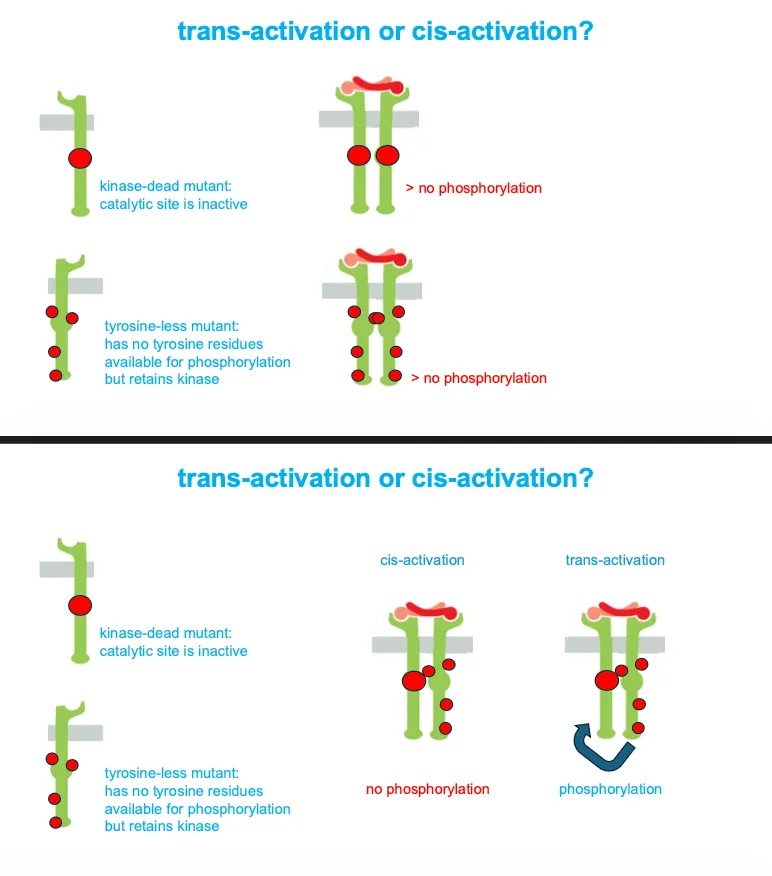
How do we know that enzyme coupled receptors work with trans-activation????
When we have a dead end mutant and a tyrosine less mutant together they would still give phosphorylation, proving that each subunit phosphorylates tyrosines on the other partner (trans) rather than on itself.
If they are mutated we would have no phosphorylation in both cases.
If they are co-activated in cis activation there would be no phosphorylation and for trans there would be.
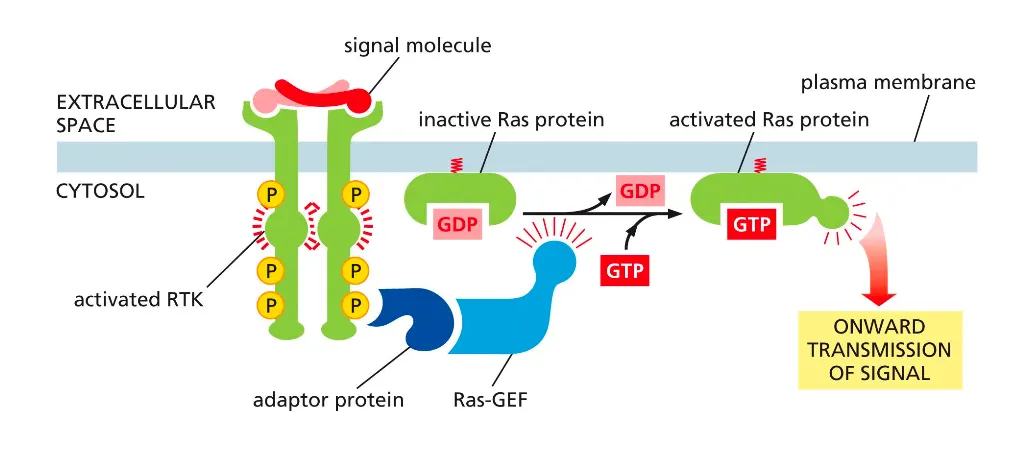
RTKs activate the monomeric GTPase Ras. How??
The broad context is growth factor signaling through receptor tyrosine kinases (RTKs): an extracellular signal activates RTKs, which trans‑autophosphorylate, recruit adaptor proteins and Ras‑GEFs, switch Ras from GDP‑bound to GTP‑bound, and thereby launch intracellular signaling cascades that control cell proliferation, survival, or differentiation.
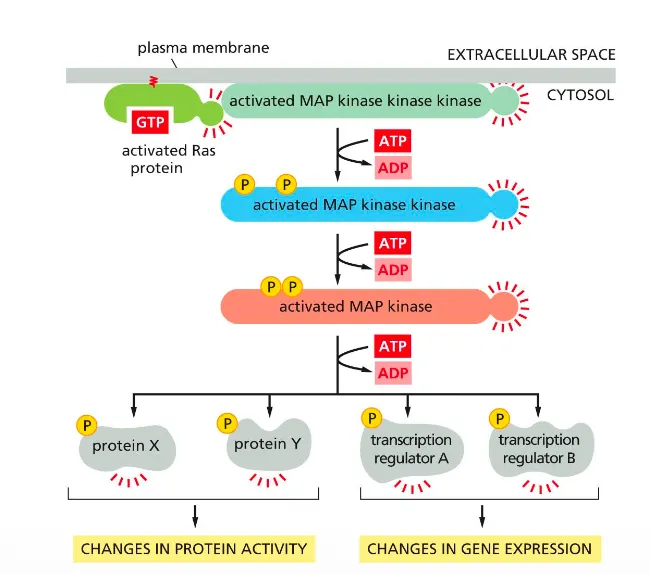
Ras activates the MAP kinase signaling pathway
This slide shows that active Ras triggers a MAP kinase phosphorylation cascade that alters protein activity and gene expression.
Ras–GTP activates a MAP kinase kinase kinase at the membrane, which phosphorylates and activates a MAP kinase kinase, which in turn activates MAP kinase.
Activated MAP kinase phosphorylates cytosolic proteins and nuclear transcription factors, producing changes in protein activity and in transcription of target genes.
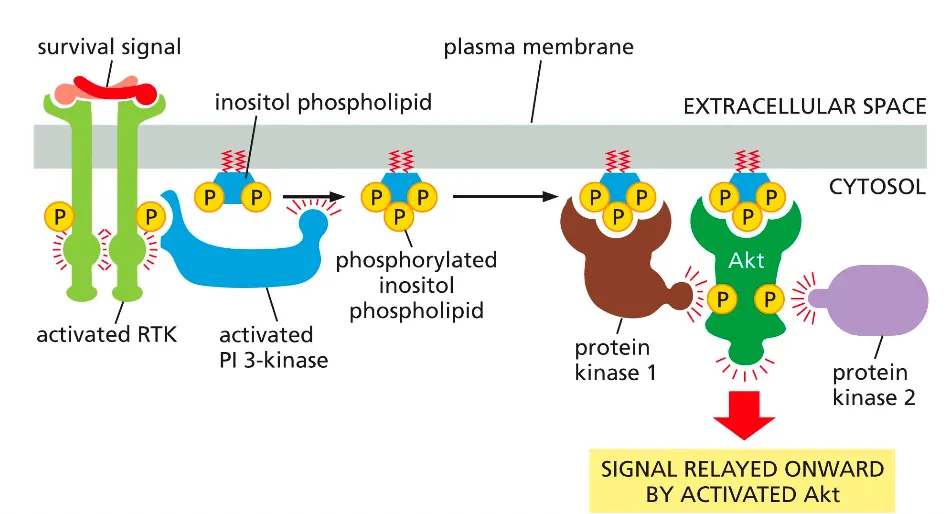
PI3K-Akt signalling pathway. How does that work??
A survival signal activates an RTK, which recruits and activates PI3‑kinase; PI3K phosphorylates a membrane inositol phospholipid to make PIP₃.
PIP₃ recruits protein kinase 1 and Akt to the membrane, where Akt is phosphorylated and activated, then goes on to phosphorylate downstream targets that promote cell survival and growth.
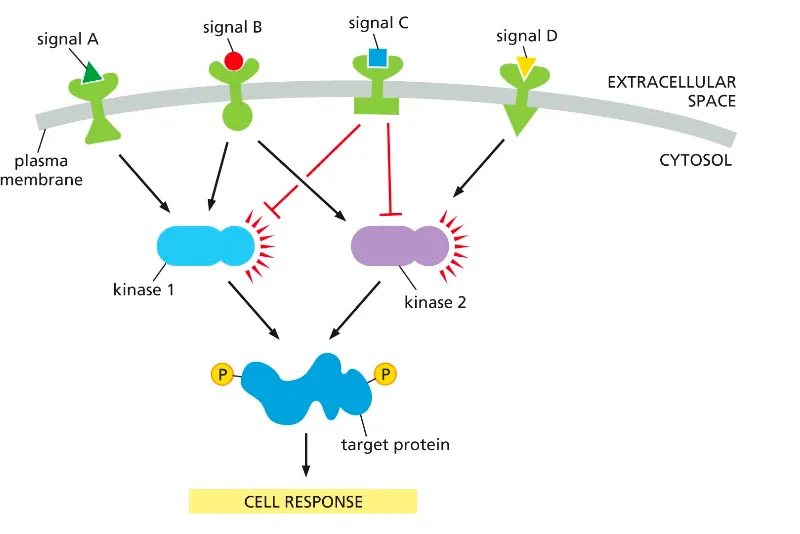
Cells integrate incoming signals using converging and diverging signaling pathways. Can you explain this mechanism??
Different receptors (signals A–D) activate or inhibit shared kinases (kinase 1 and kinase 2), which can both converge on the same target protein.
The balance of activating and inhibitory inputs on these kinases determines the phosphorylation state of the target and thus the overall cell response.
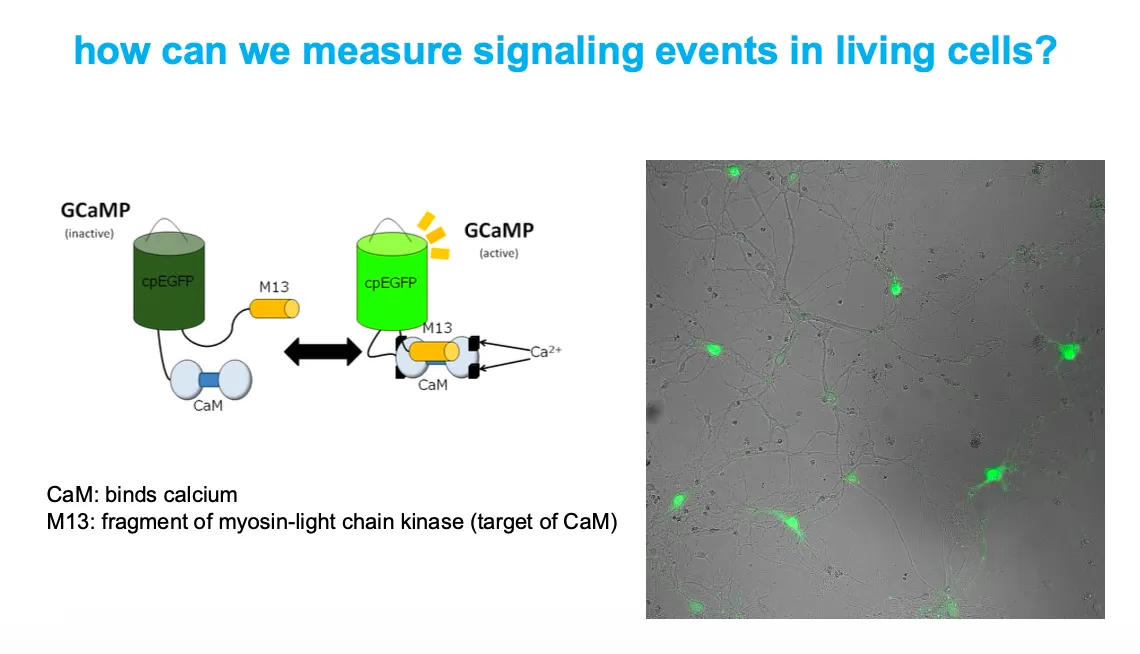
How can we reports signaling events in living cells
GCaMP is a fusion of circularly permuted EGFP, calmodulin (CaM), and an M13 peptide; when Ca²⁺ binds CaM, CaM–M13 clamp around cpEGFP and increase its fluorescence.
Cells expressing GCaMP light up green in regions where intracellular Ca²⁺ rises, allowing real‑time imaging of calcium‑dependent signaling.