MC-carbonyl Chemistry
1/32
Earn XP
Description and Tags
the group,its nomenclature its properties,reacivity and its type of reactions
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
33 Terms
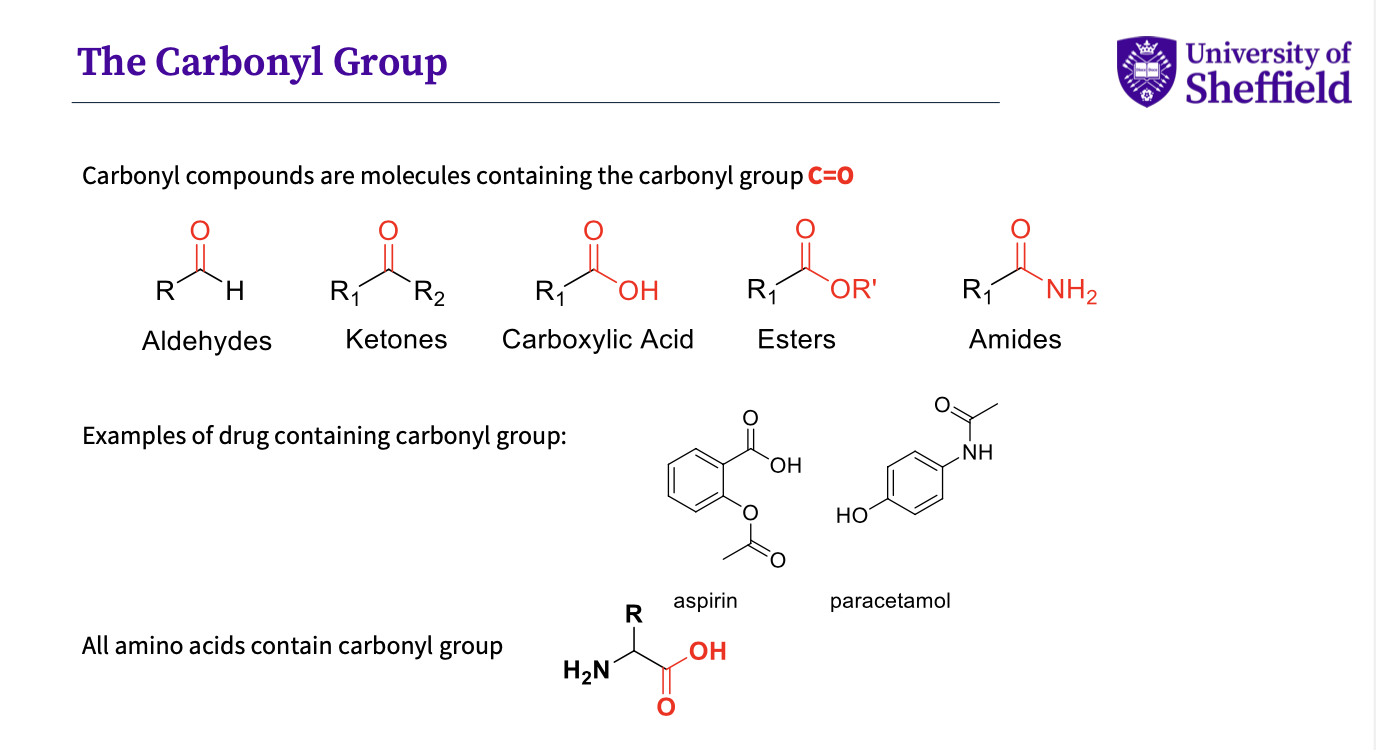
What is a carbonyl compund?
-Something that contains a carbonyl group c=o
eg . aldehydes,ketones,carboxylic acid,esters,
drug containing carbonyls - aspirin,paracetomol
note- all amino acids contain carbonyl group
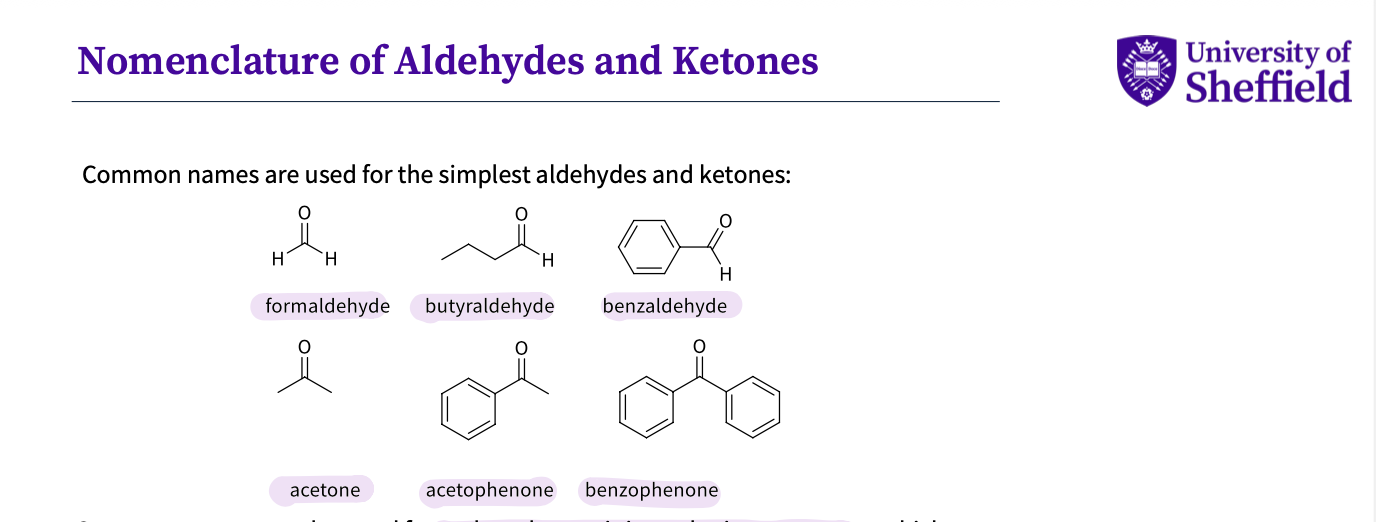
What are some common names used to describe simple ketones and aldehydes?
-formaldehyde- 2 H
-butyraldehyde - 4 carbon
-benzaldehyde- benzene ring
-acetone- normal 3C
-acetophenone - benzene and 3 C
-benzophenone - 2 benzene rings
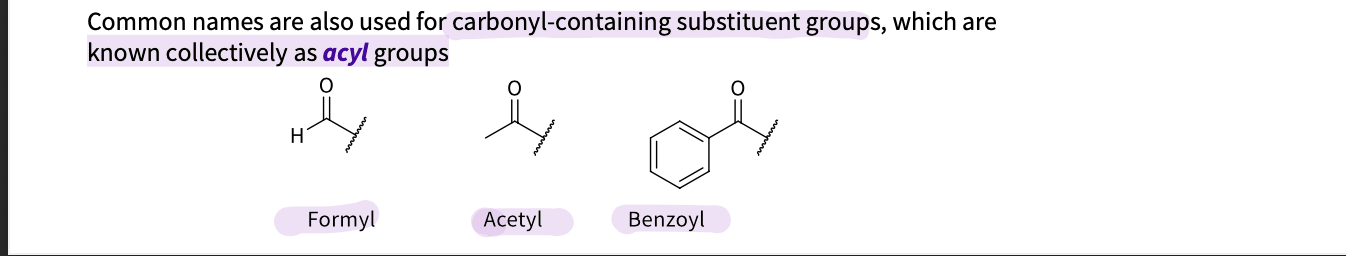
What are some common names used to describe carbonyl-containing substituent groups ?
collectively known as acyl groups
formyl- 1 hydrogen and a chain
acetyl - no hydrogen and a chain
Benzoyl- benzene ring and a chain
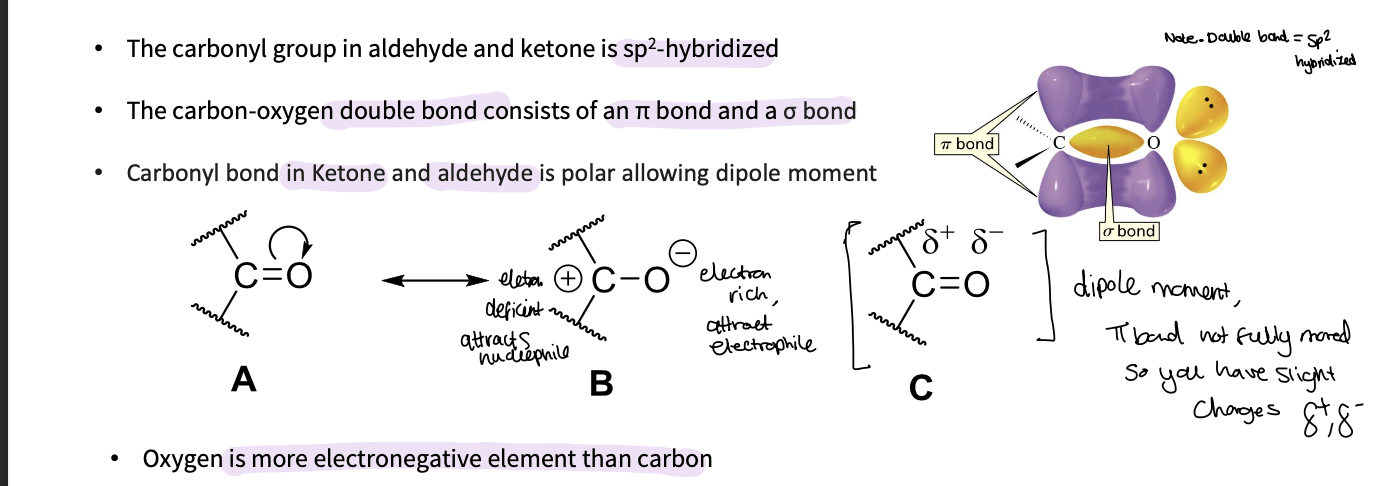
What are some properties of the carbonyl bond?
in an aldehyde and ketone it is sp2 hybridized
The carbon-oxygen double bond consists of an π bond and a σ bond
• Carbonyl bond in Ketone and aldehyde is polar allowing dipole moment ( the π bond isn’t fully moved so you have slight charges )
as oxygen is more electronegative than carbon
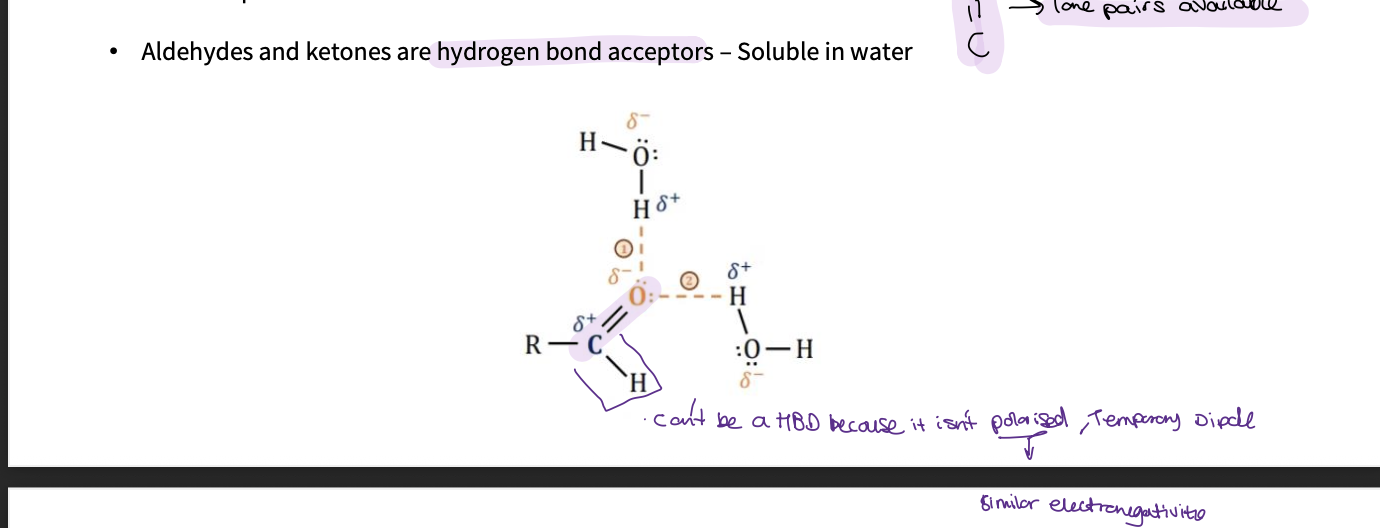
Why are aldehydes and ketone HBA but not HBD?
they can’t donate a proton
the C-H bond isn’t polarised as the carbon and hydrogen have similar ELECTRONEGATIVITIES - so only temporary induced dipole
they are HBA as the 2 lone pairs on oxygen is available - this makes them soluble in water
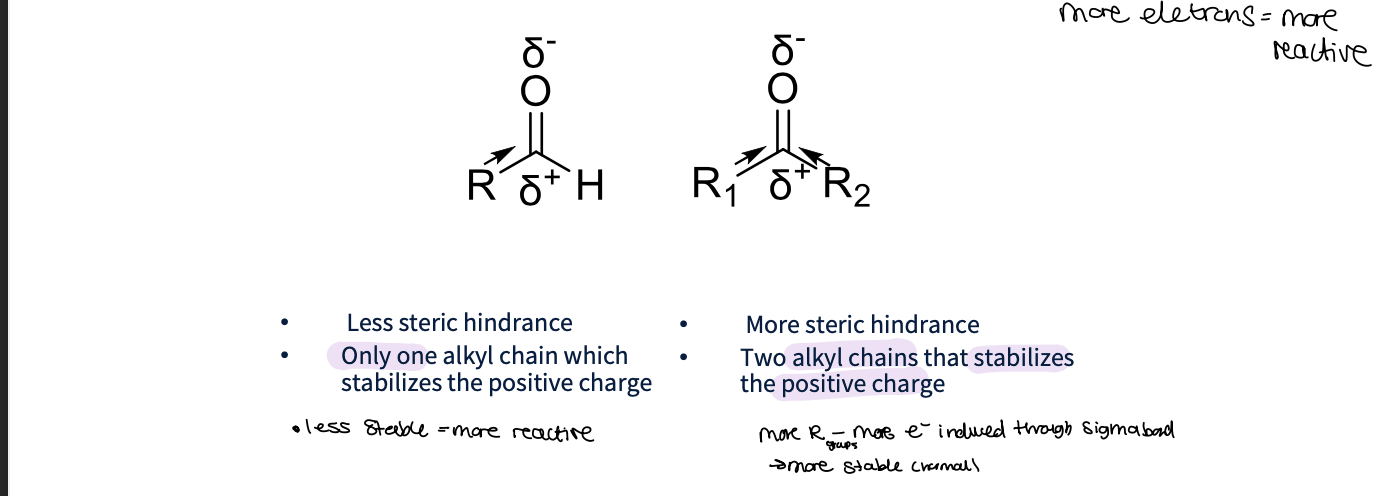
Why are Aldehydes more reactive than ketones ?
In aldehydes 🇦:
Less steric hindrance
Only one alkyl chain which stabilizes the positive charge
less stable as less alkyl groups lower electron releasing effect so = more reactive
In Ketone:
More steric hindrance
• Two alkyl chains that stabilizes
the positive charge
more R groups = more Electrons induced through sigma bonds = more stable
Why can carbonyl bonds do nucleophilic and electrophilic reactions ?
Due to carbonyl bond polarisation, carbonyl group is involved in nucleophilic and electrophilic reactions
the carbon has a delta + charge ( electrophilic carbon - electron poor)
the oxygen has 2 lone pairs and a delta - charge (nucleophilic oxygen - electron rich )
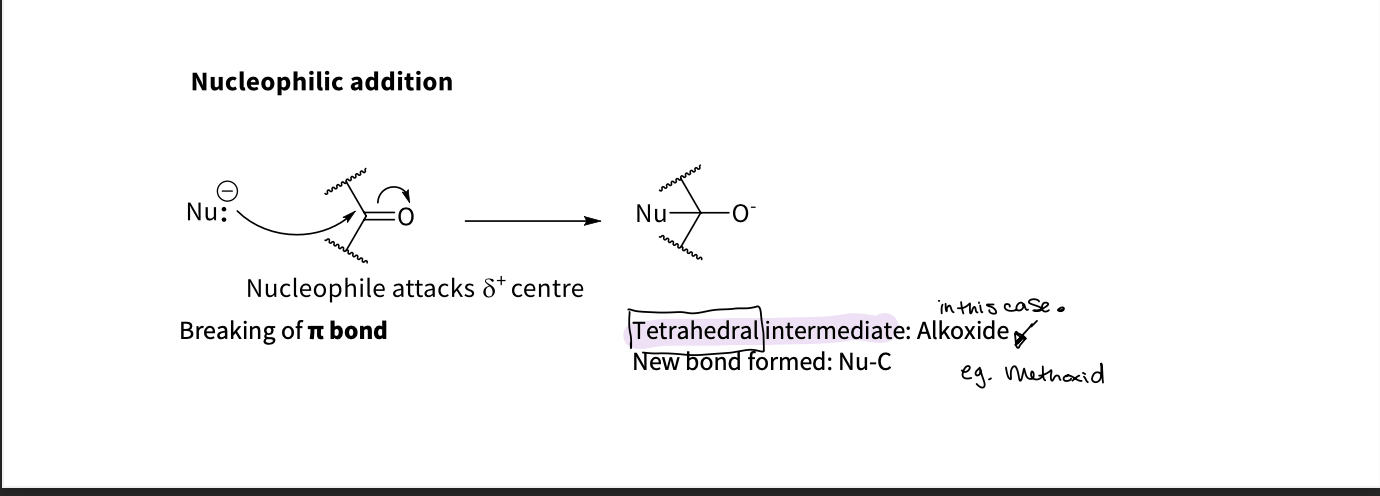
How does the carbonyl group do nucleophilic addition ?
nucleophile attacks delat = centre ,
breaking the π bond
the tetrahedral intermediate - forms as a new bond is formed between the Nu - C
this is called alkoxide ( eg methoxide etc)
How can carbonyl groups do electrophilic addition ?
oxygen lone pair attacks electrophile
the oygen now has a + charge = 3 bonds Formation of oxonium intermediate
one of the double bonds break and carbon now has a + charge
Note - most addition involves both steps,the order in which these occurs depends on the nature of the reagent and reaction conditions ( The Ph )
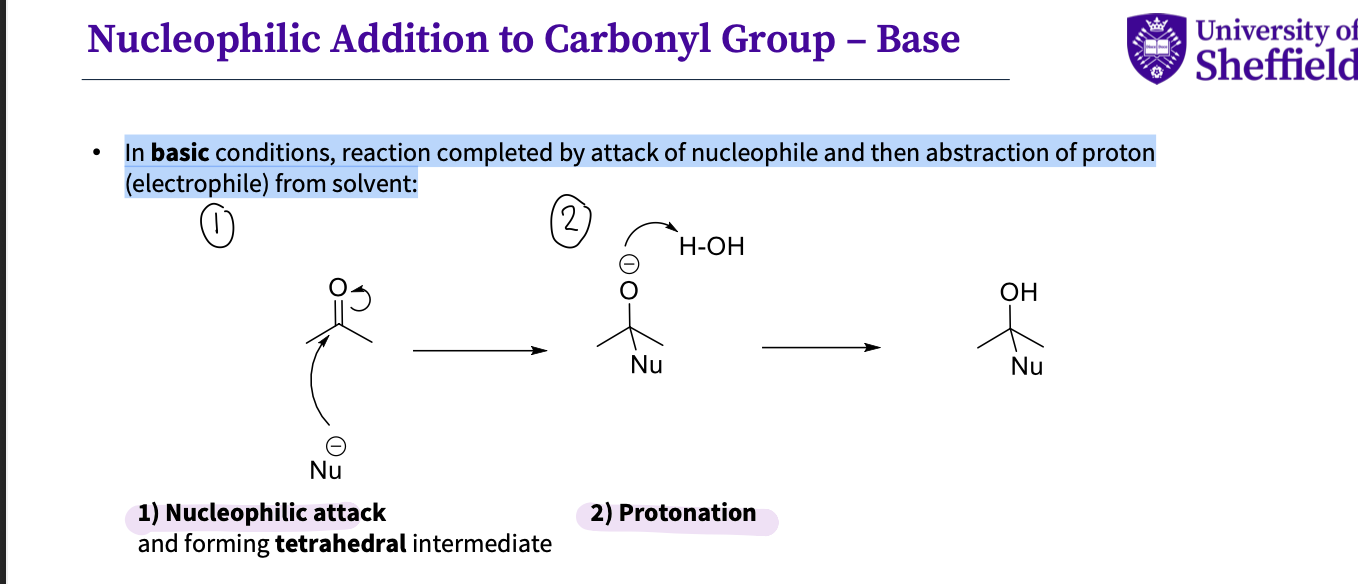
What changes when nucleophilic addition happens in basic conditions?
In basic conditions, reaction completed by attack of nucleophile and then abstraction of proton
(electrophile) from solvent:
nucleophilic attack = a tetrahedral intermediate
protonation to oxygen thi is called nucleophilic as the most significant change is formation of C-Nu
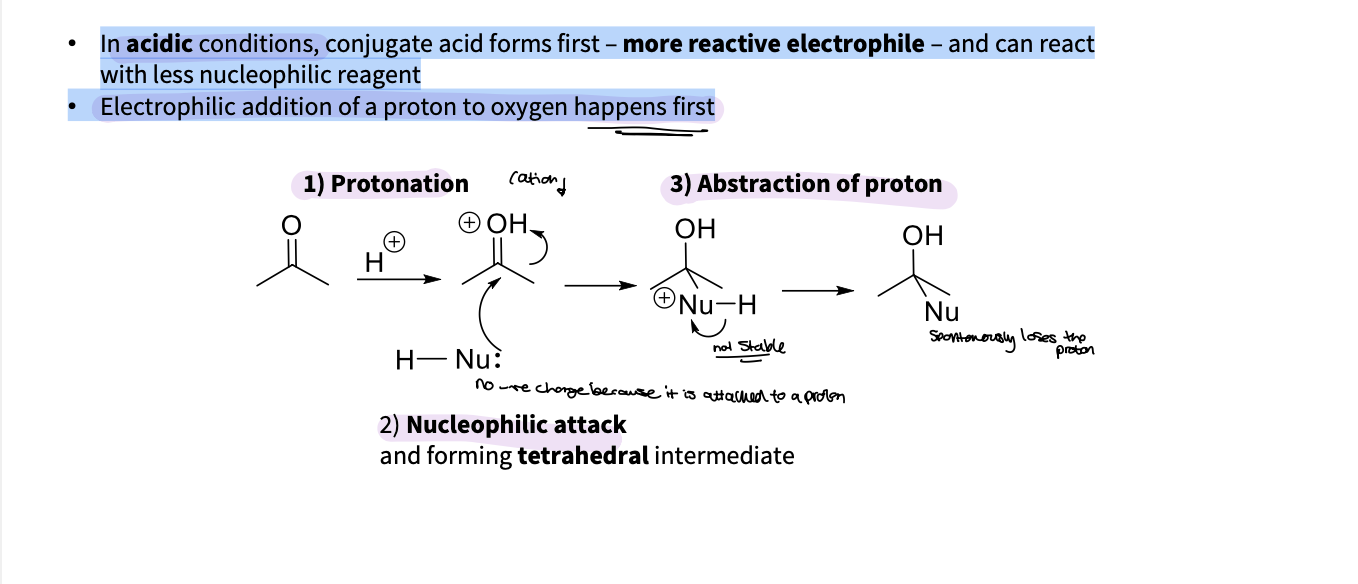
What changes when nucleophilic addition happens in acidic conditions ?
In acidic conditions, conjugate acid forms first – more reactive electrophile – and can react
with less nucleophilic reagent
• Electrophilic addition of a proton to oxygen happens first
protonation
nucleophilic attack - tetrahedral intermediate
abstraction of proton
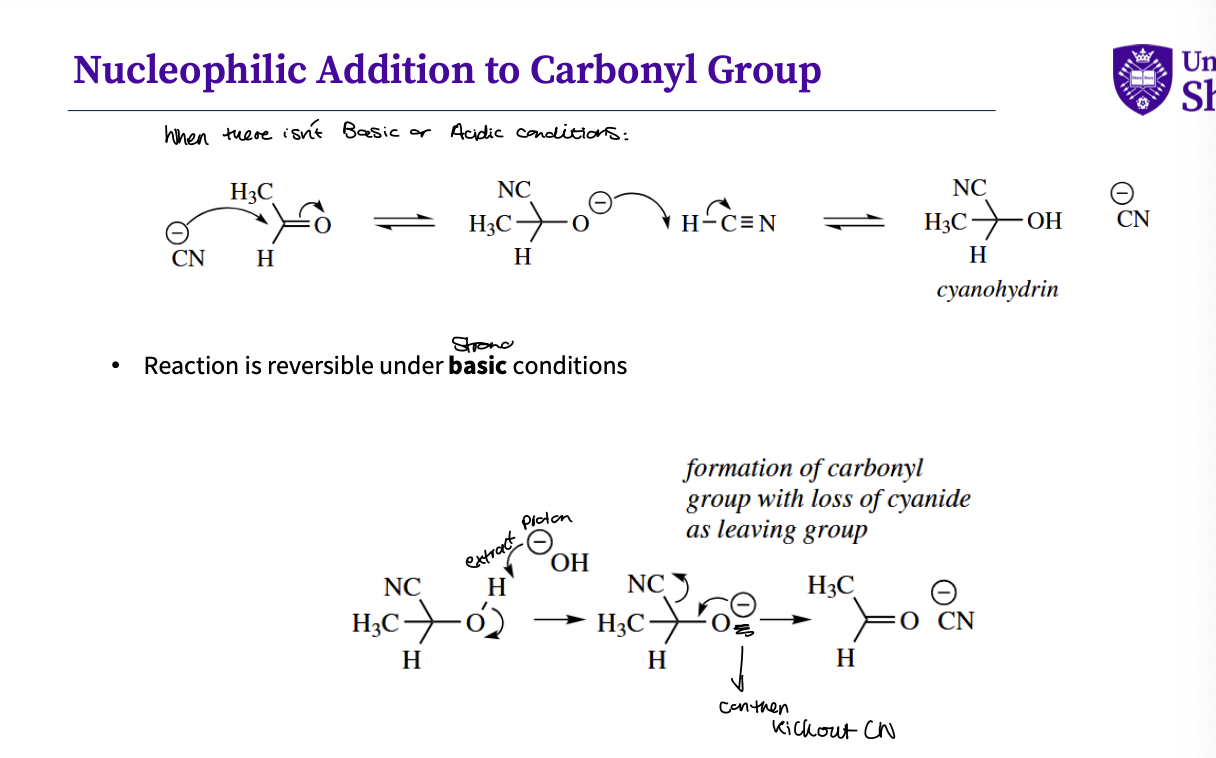
Example - normal conditions of nucleophilic addition
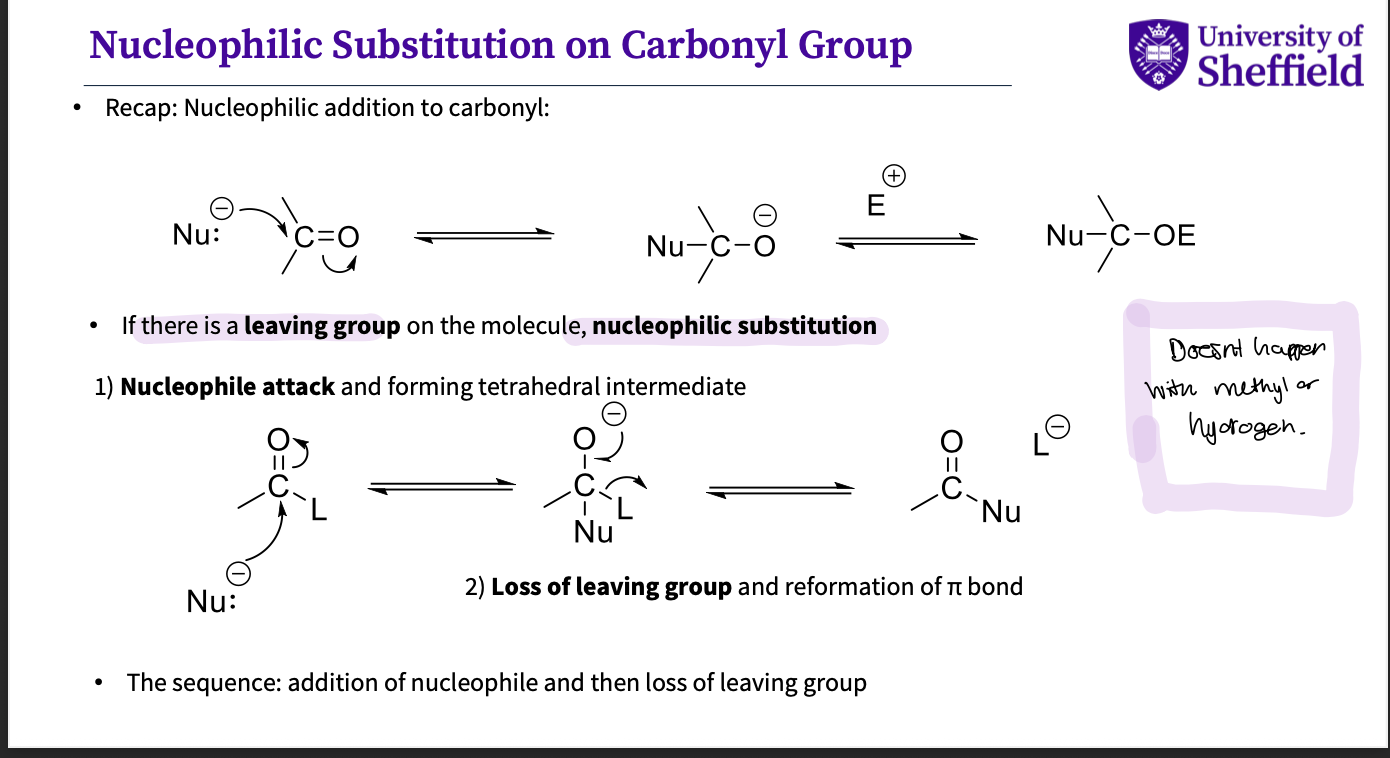
Why do we have nucleophilic substitution instead of addition ?
if there is a leaving group = substitution not with methyls or hydrogens
nucleophile attack and forming a tetrahedral intermediate
loss of leaving group - and reformation of π bond
summary - addition of nucleophile,loss of leaving group
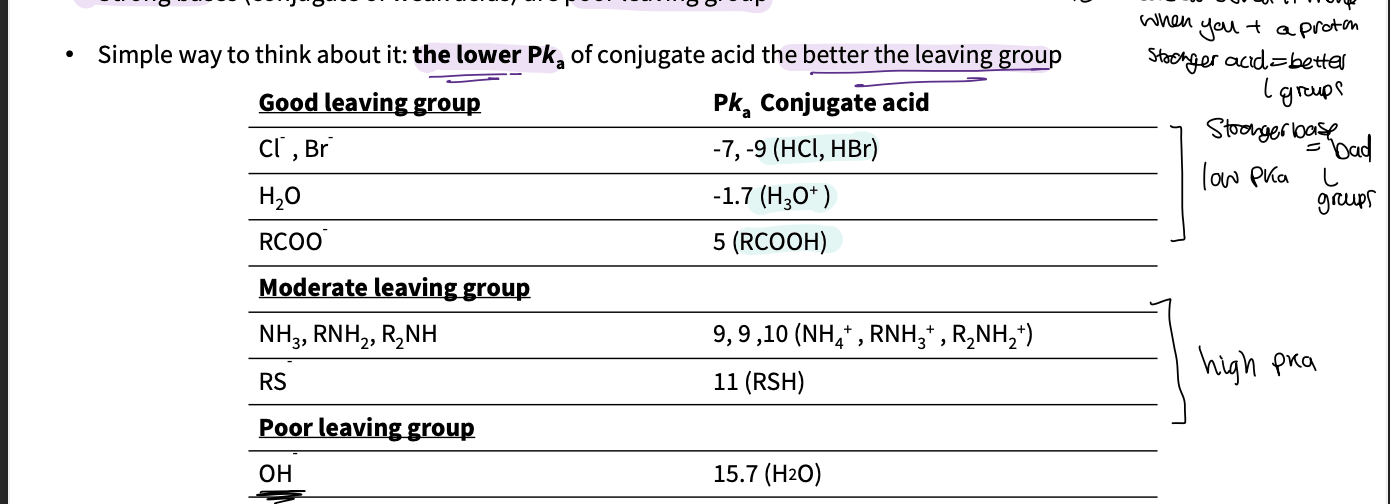
What makes a good leaving group ?
lower pka of conjugate acid ( what is made if you add a proton to L group)= better leaving group
eg weak bases = good l groups
strong bases=poor l groups
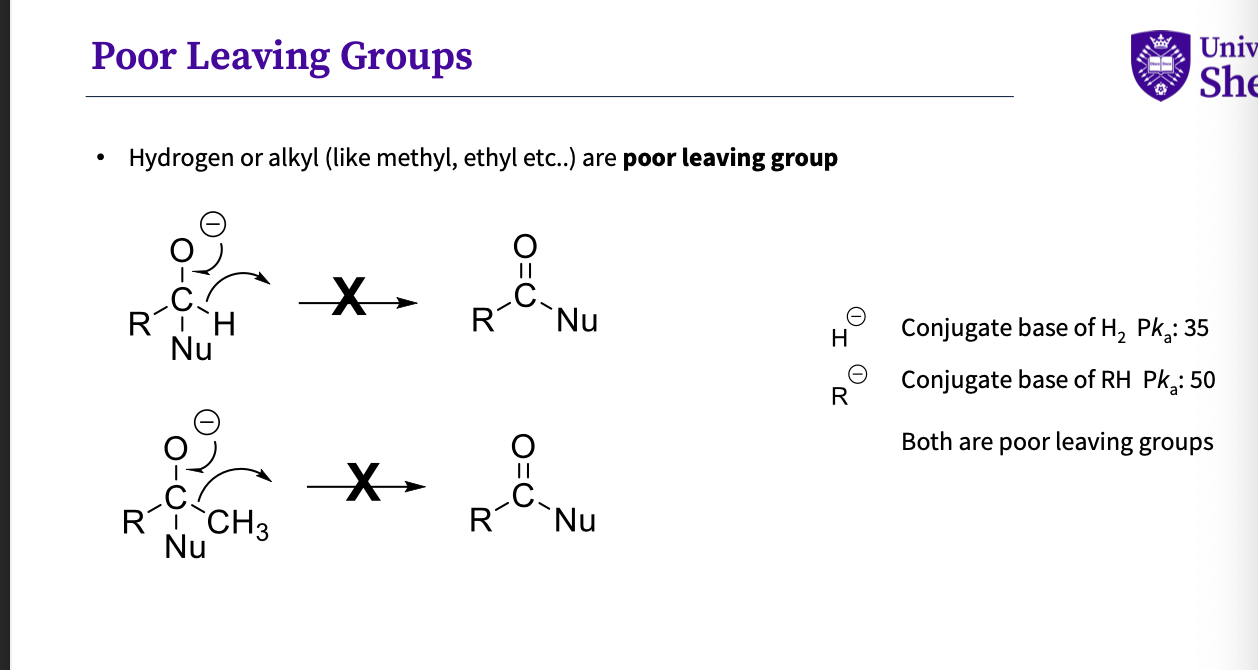
What are bad leaving groups ?
hydrogen or alkyl ( like methyls,ethyls) are poor leaving groups
this is because the conjugate base for h2 = h- high pKa = 35
for rH the conjugate base = R- high pKa = 50
so are therefore poor leaving groups
Give an example of a leaving group ?
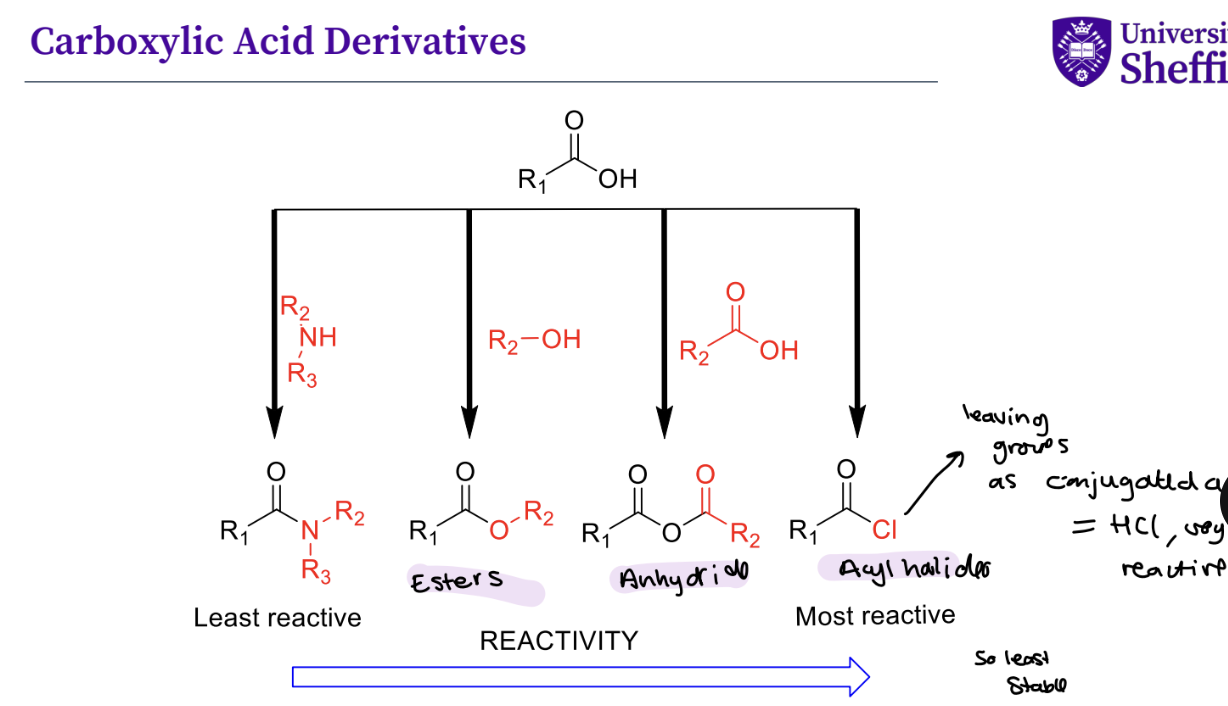
Acyl Halides - really important
carboxylic acid derivatives - have better leaving groups
acyl halides - can form esters ,and form amides
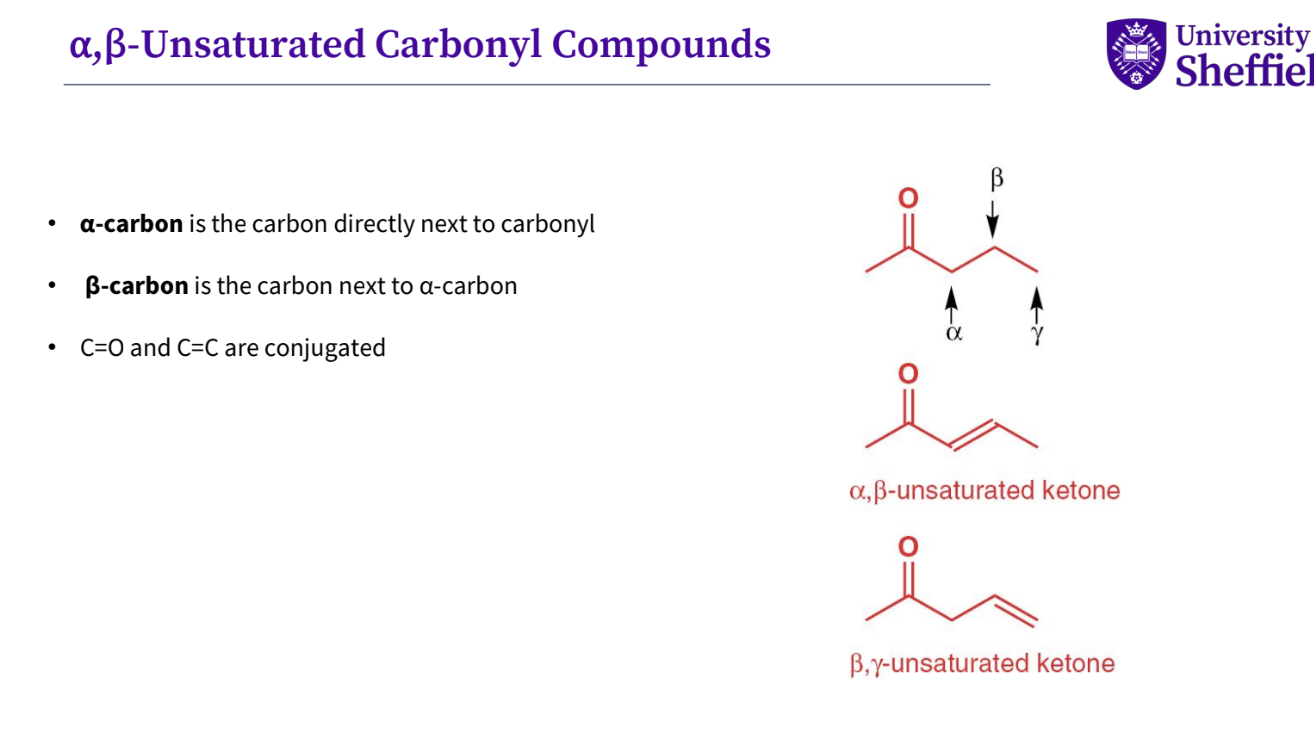
What is a ⍶ , ϐ unsaturated carbonyl compound ?
α-carbon is the carbon directly next to carbonyl
• β-carbon is the carbon next to α-carbon
• C=O and C=C are conjugated
why - the conjugated system is reactive for drug design
What are the properties of ⍺,β-Unsaturated Carbonyl Compounds?
The conjugation changes the electronic properties of the alkene
• In the conjugated system, alkenes are electrophiles
• The carbonyl pulls electron density away through resonance, making the alkene electron-poor and therefore electrophilic.
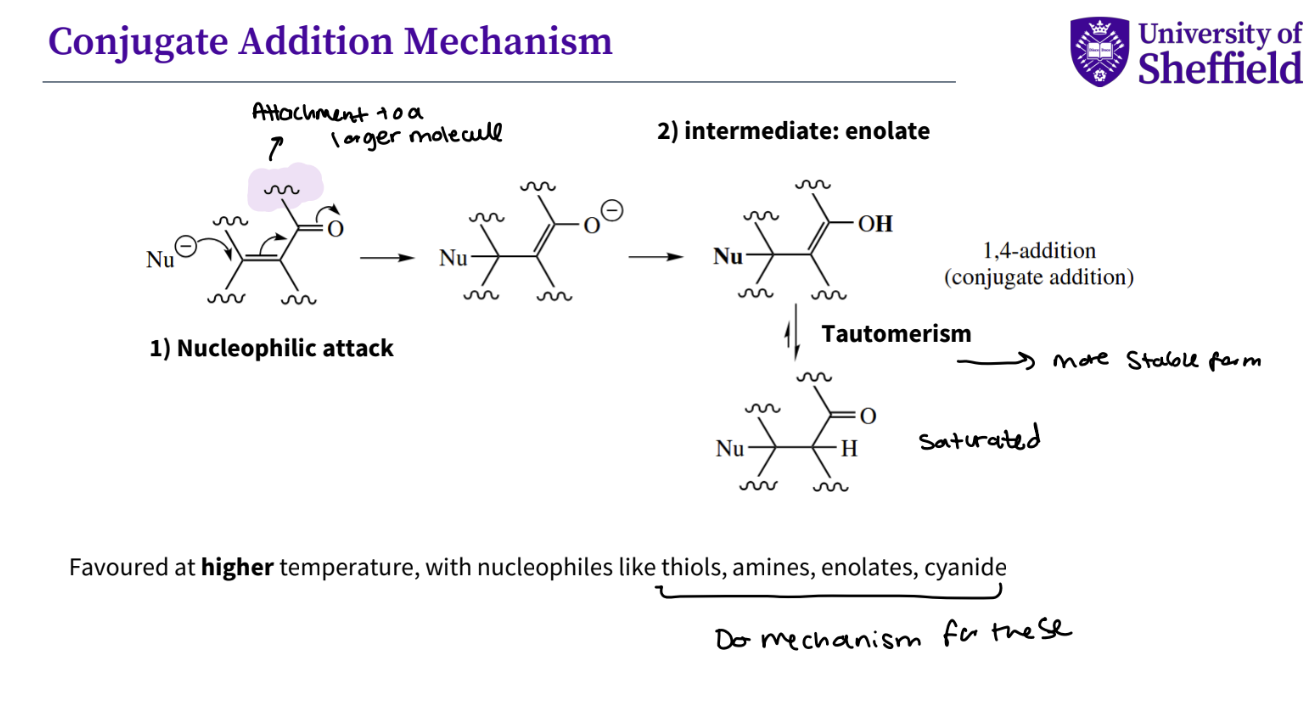
What is a conjugate addition mechanism ?
nucleophilic attack , the double bond breaks and the oxygen double bond breaks , so the oxygen has a negative charge
intermediate - enolate -a resonance-stabilized anion formed by removing a proton from the alpha-carbon (the carbon next to a carbonyl group) of an organic compound
then there is tautomerism - more stable form a type of structural isomerism where a molecule can exist in two or more interconvertible forms called tautomers, becaus ethe protons move
you then have a saturated compound
note- this is favoured at a higher temperature , which nucleophiles like thiols , amines , enolates , cyanide
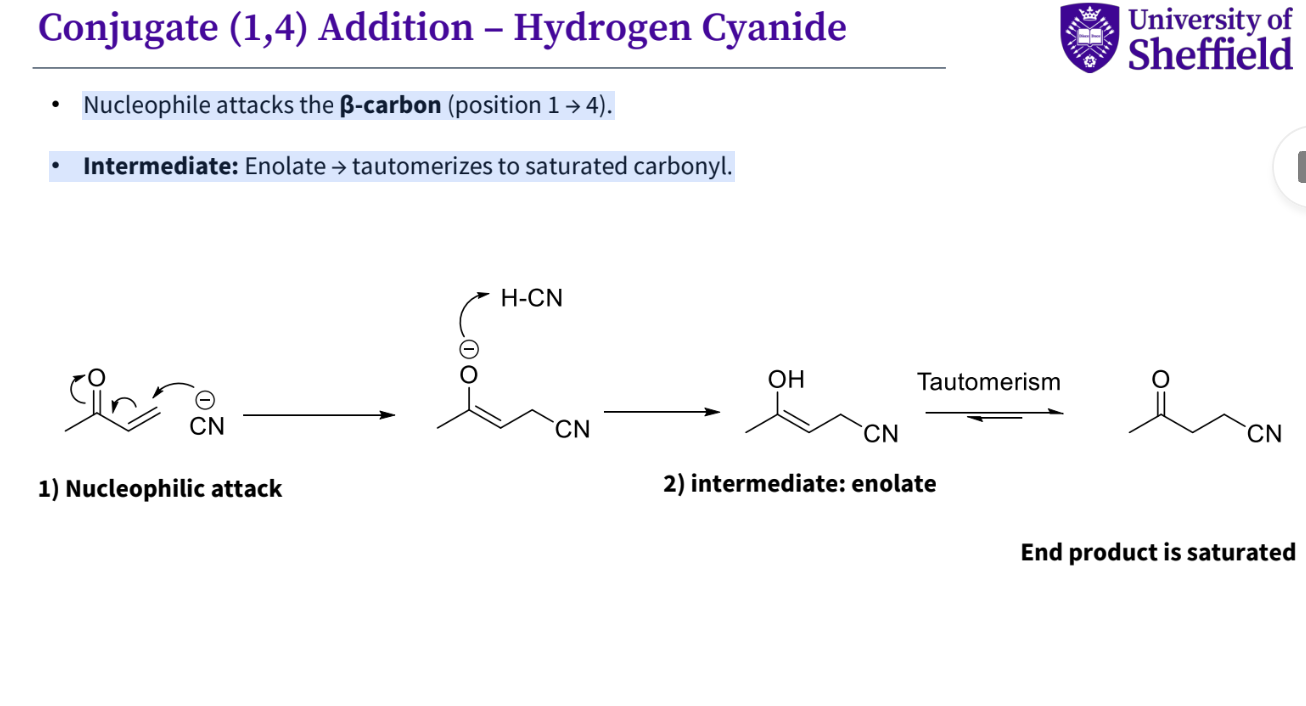
Example - conjugate addition - hydrogen cyanide
Nucleophile attacks the β-carbon (position 1 → 4).
• Intermediate: Enolate → tautomerizes to saturated carbonyl.
watch a video :
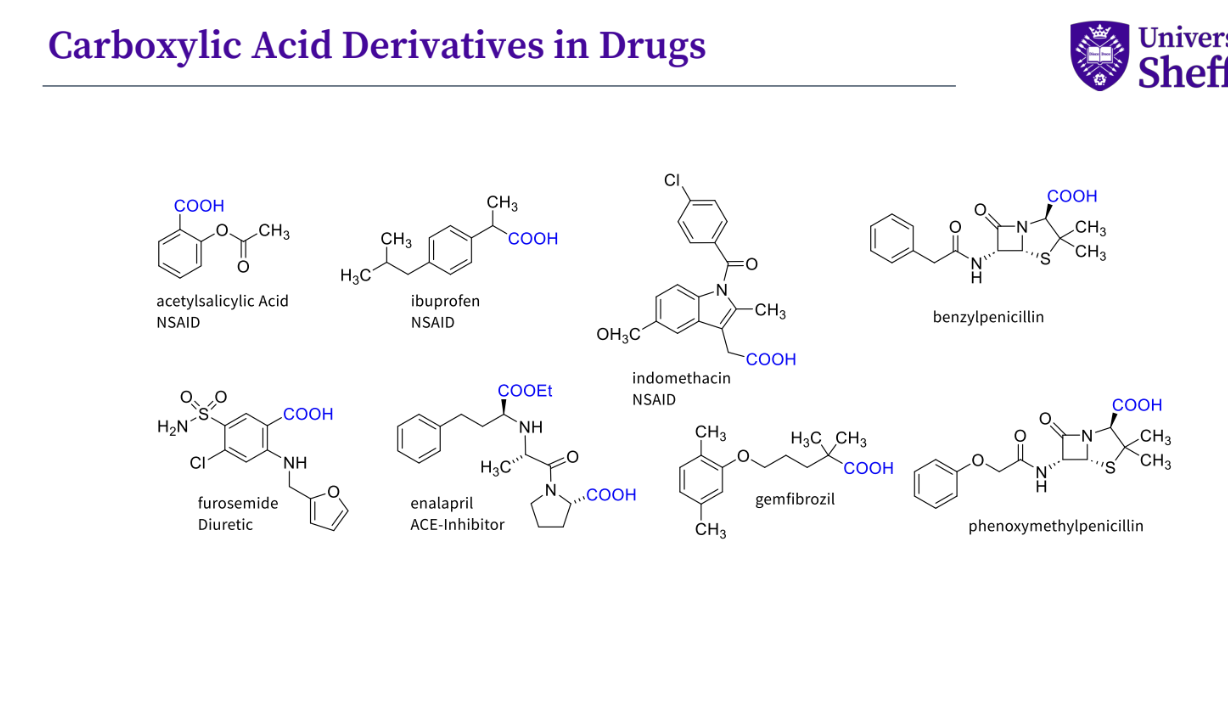
What are some common carboxylic acid derivatives ?
What are key carboxylic acid derivatives and their reactivity ?
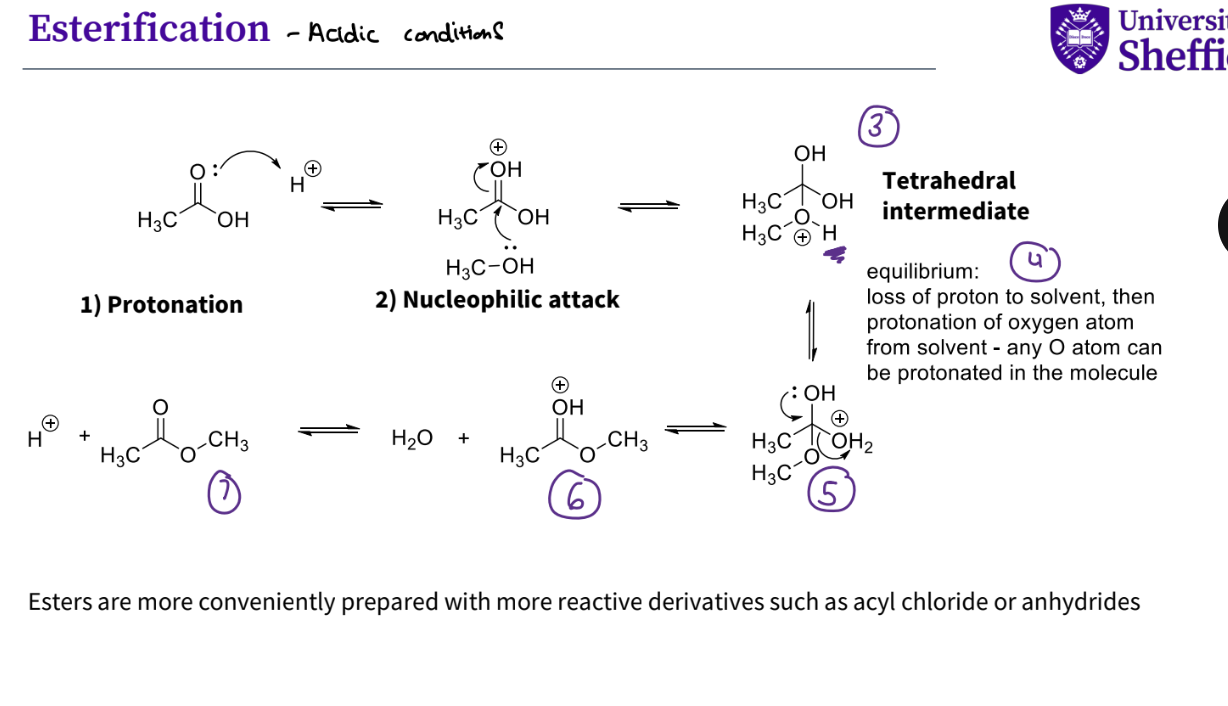
What are the key steps in esterification - in acidic conditions ?
protonation - lone pair attacks a proton ,it forms a bond with oh , oxygen now has a + ve charge because it is forming too many bonds
nucleophilic attack, lone pair on the oh on an alcohol attacks the carbon on carbonyl group , the double bond breaks to squash the positive charge on oxygen
a tetrahedral intermediate is formed , there is a + ve charge on the oxygen that has just attached to the carbon
there is an equilibrium , the proton is lost to the solvent , then protonation of the oxygen atom from solvent , any oxygen atom can be protonated in the molecule
the lone pair on the oh forms a double bond and the oh2 ( protonated oxygen that has a + ve charge ) leaves
there is still a positive charge on the hydroxyl oxygen so that leaves
you have your ester and H +
ESTERIFICATION - general equation
Alcohol + Carboxylic acid —→ ester + water
Note - esters are more conveniently prepared with more reactive derivatives such as acyl chlorides or anhydrides
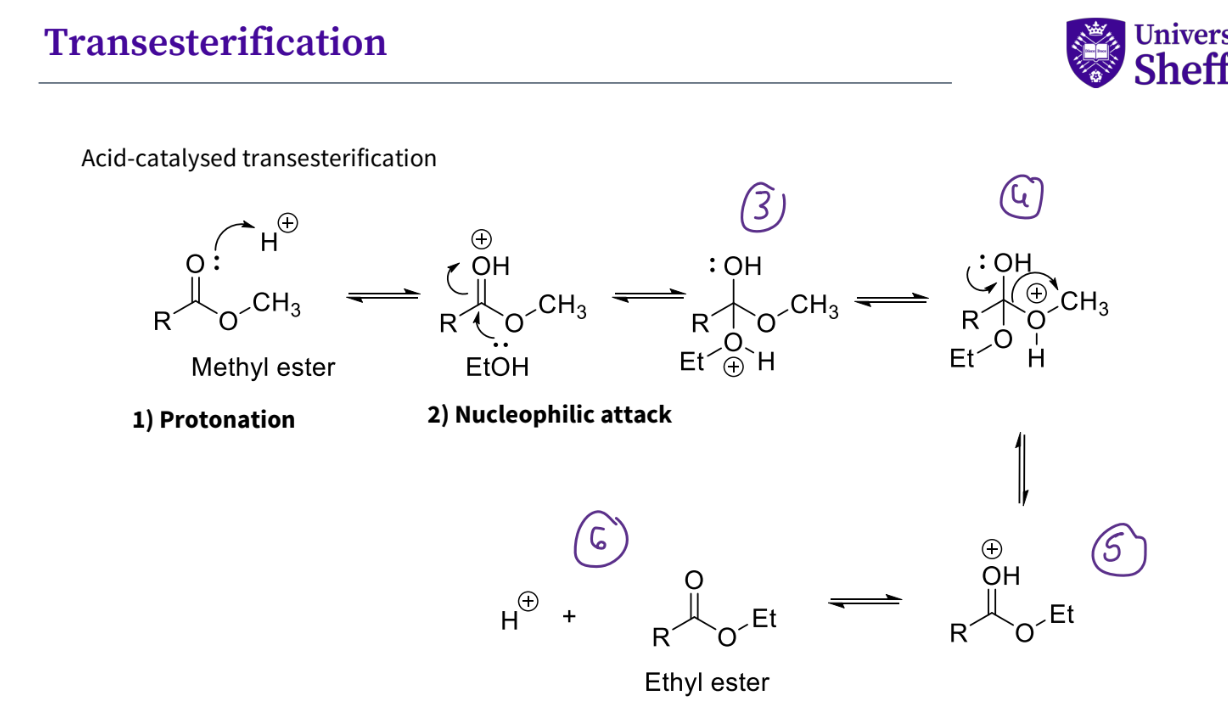
What is transesterification ?
A chemical reaction where an ester and an alcohol swap their alkyl groups, typically in the presence of a catalyst.
Describe what happens in acid- catalysed transesterification ?
protonation of the lone pair on the oxygen
nucleophilic attack of the etOH on the carbonyl,the oH double bond breaks
there is now a + ve charge on the oxygen , the hydrogen is then kicked off- by tautomerism
the bond breaks removing the + vely charged oxygen group , and a new bond is formed between the OH the carbon.- a double bond
there is now a + vely charged oxygen , with a hydrogen with a double bond
the hydrogen - proton leaves forming its ester
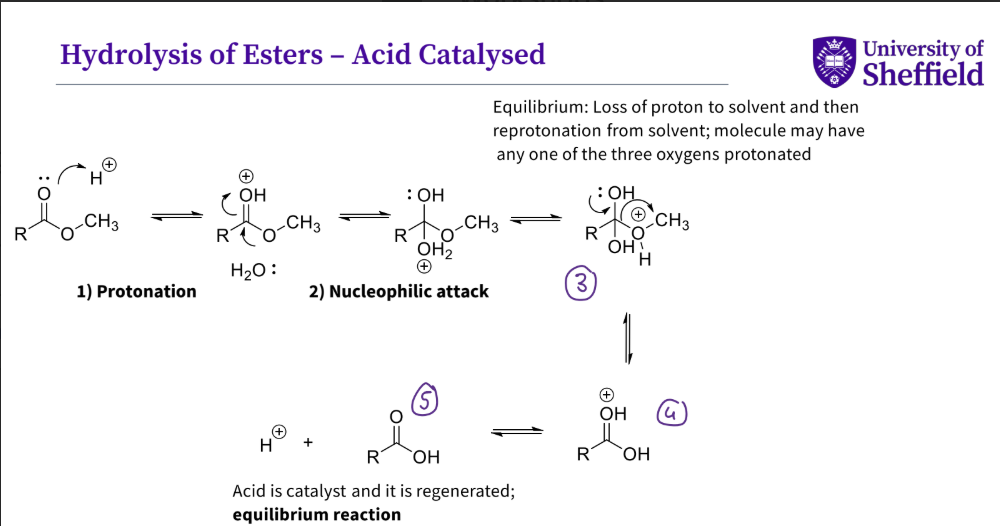
What is the mechanism for hydrolysis of esters - acid catalysed ?
protonation
nucleophilic attack by water , hydrogen is lost
the double bond reforms and the alcohol group is kicked out
there is a positive charge on double bonded hydroxyl groups , hydrogen leaves
the alcohol and proton is regenerated
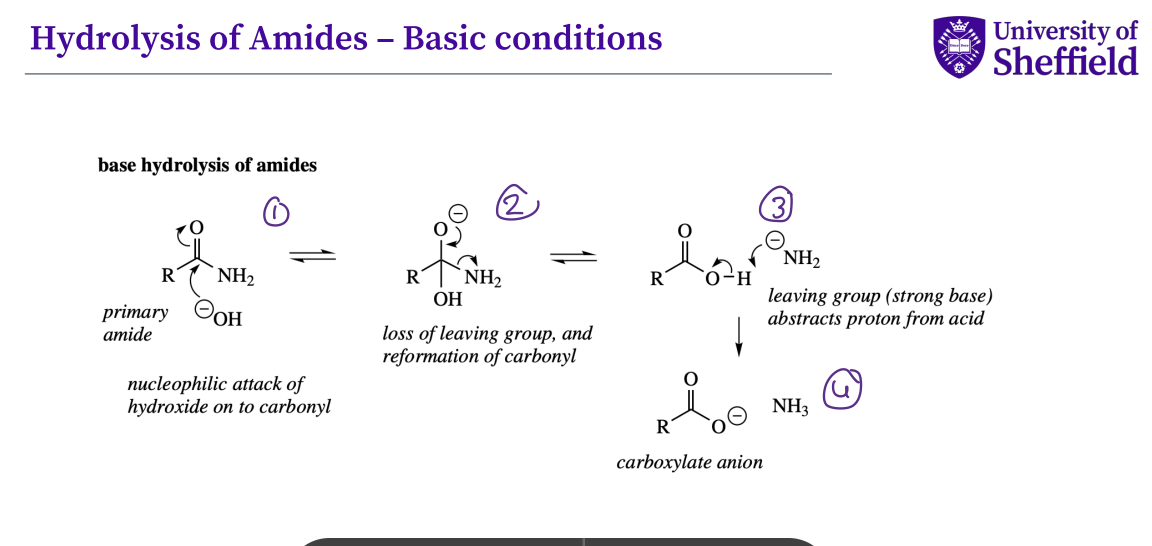
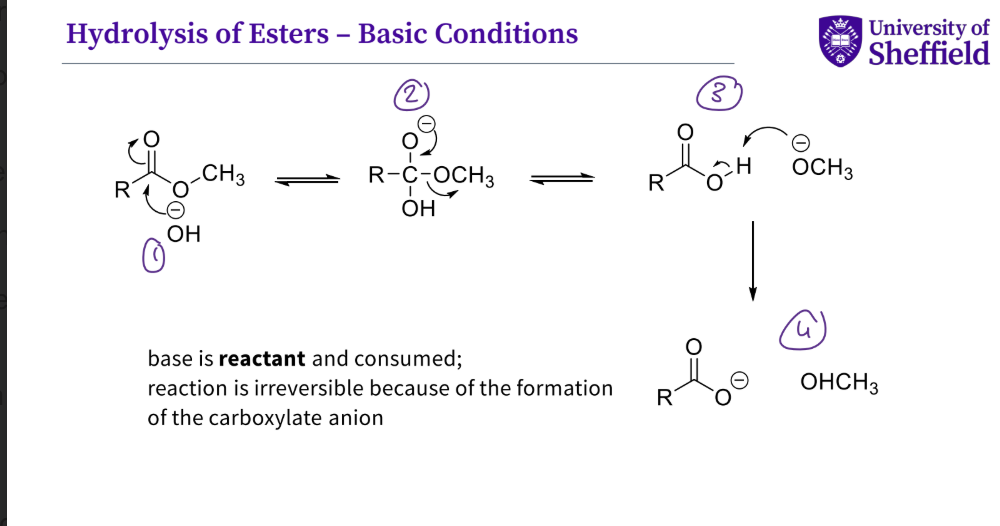
What is the mechanism for the hydrolysis of esters under basic conditions ?
the hydroxyl group attacks the carbonyl , the double bond O breaks
the lone pair of electrons refirm the double bond , the single oxygen bond breaks
the - vely charged group now attack the hydrogen which then causes the bond to break
a carboxylate anion is formed and an alcohol
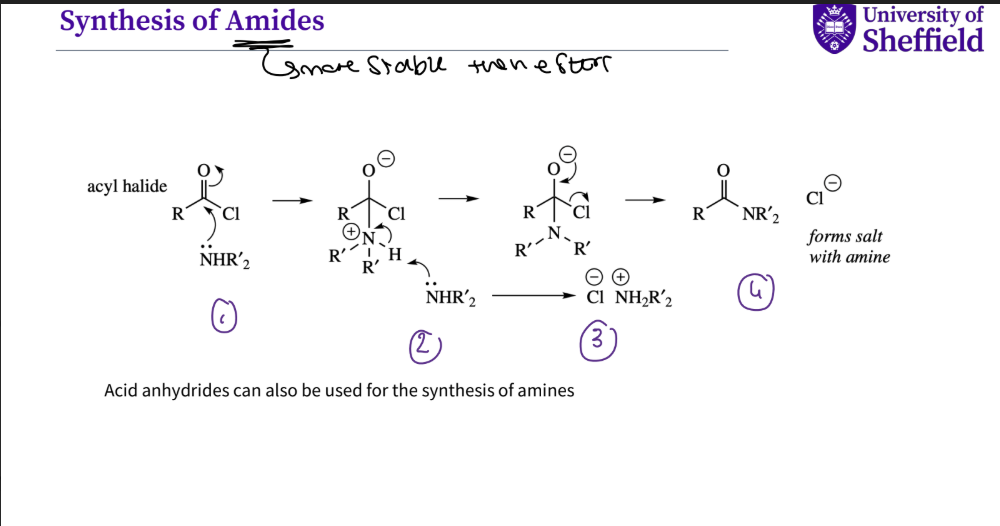
What is the mechanism for the synthesis of amides ?
Amides are more stable then esters
1 .an amine attacks the carbonyl on the acyl halide the double bond breaks
a second amine attacks the hydrogen which then squashes the + ve charge
the oxygen then reforms its double bond , kicks out halide , and a salt is formed
amide is formed and halide ion
note- acid anhydrides can also be used for the synthesis of an amide
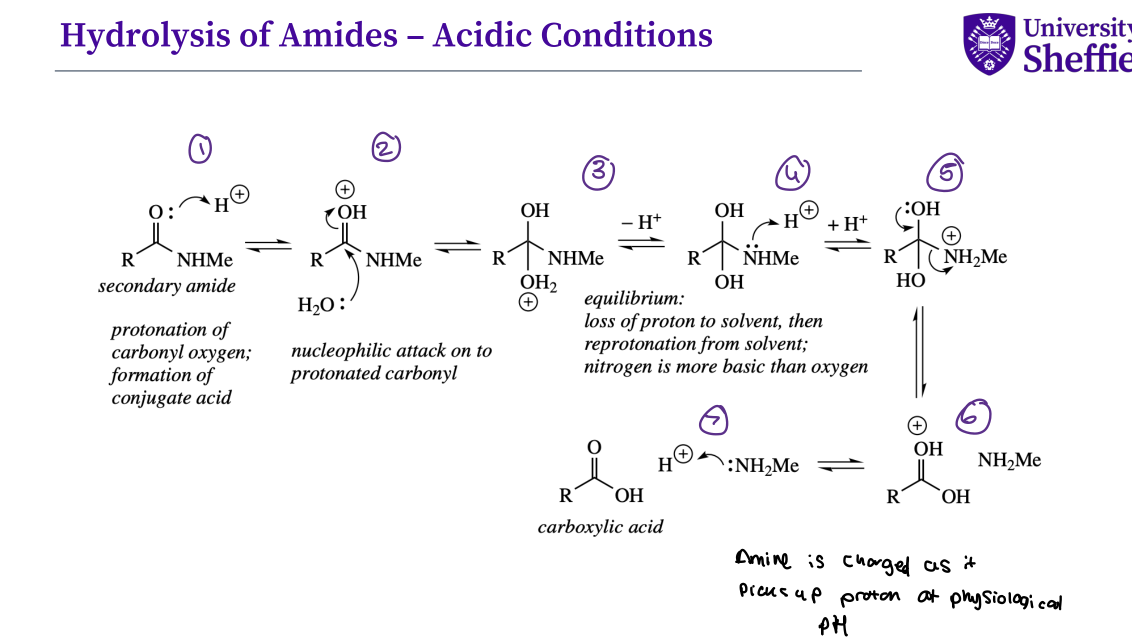
Compare the hydrolysis of amides vs esters leaving groups ?
Amides are hydrolysed under basic or acidic conditions:
• The reaction is significantly slower compared to ester hydrolysis
• The leaving group in case of ester (-OR) is far better leaving group compared to amide (-NHR)
• -NHR is far stronger base (weaker leaving group ) compared to -OR
Note - amides are hard to hydrolyse
Leaving Conjugate Ac PKa group:
-OR ROH 15
-NHR NH2R 35
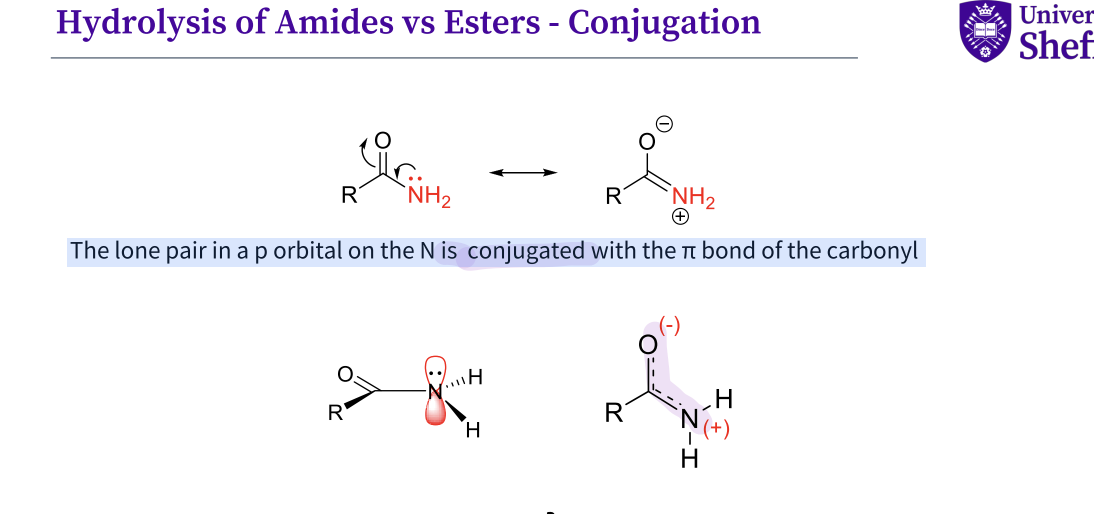
What is the conjugation in the hydrolysis of amides and esters ?
The lone pair in a p orbital on the N is conjugated with the π bond of the carbonyl
amides are more stubborn - it has a hard leaving group nh2
due to it being a poor leaving group as PKA = 35 , strong base
The NH2 is conjugated with the π bond of the carbonyl
What is the mechanism for the hydrolysis of amides - acidic conditions ?
What is the mechanism for the hydrolysis of amides under basic conditions ?