Lecture 3 - Graded Potentials, Action Potentials and Synapses
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
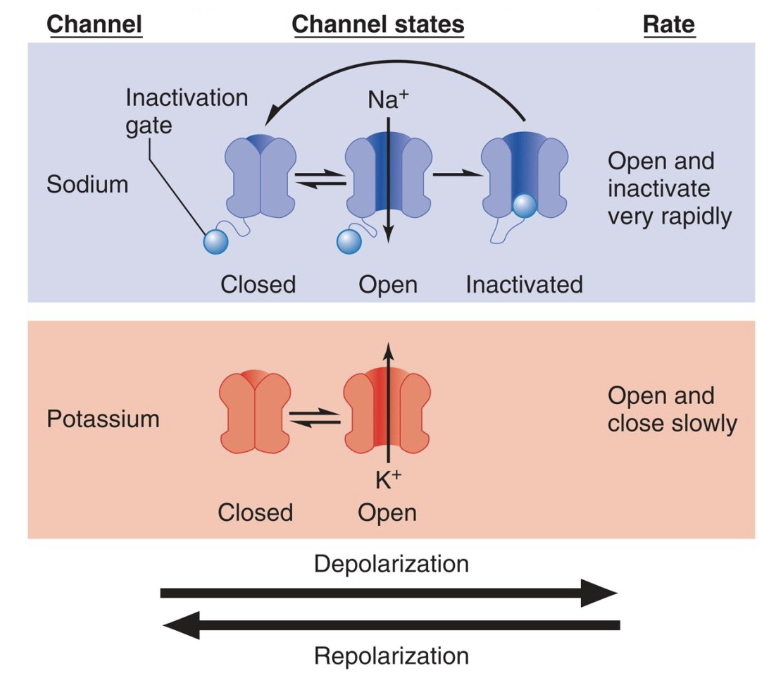
Types of Gated Ion Channels
Chemically gated (ligand-gated) channels
Voltage-gated channels
Mechanically gated channels
Chemically gated (ligand-gated) channels
Open only with binding of a specific chemical (example: neurotransmitter)
Voltage-gated channels
Open and close in response to changes in membrane potential
Mechanically gated channels
Open and close in response to physical deformation of the membrane, as in sensory receptors
When gated channels open, ions diffuse quickly along
The chemical concentration gradients from higher concentration to lower concentration
Along electrical gradients toward opposite electrical charge
Changes in Membrane Potential occurs when
(1) Ion concentrations change
(2) permeability to ions (channel opening) changes
Depolarization
Decreased membrane potential (toward zero)
Inside membrane less negative
AP probability increases
Hyperpolarization
Increase in membrane potential (further from zero)
Inside of membrane more negative
AP probability decreases
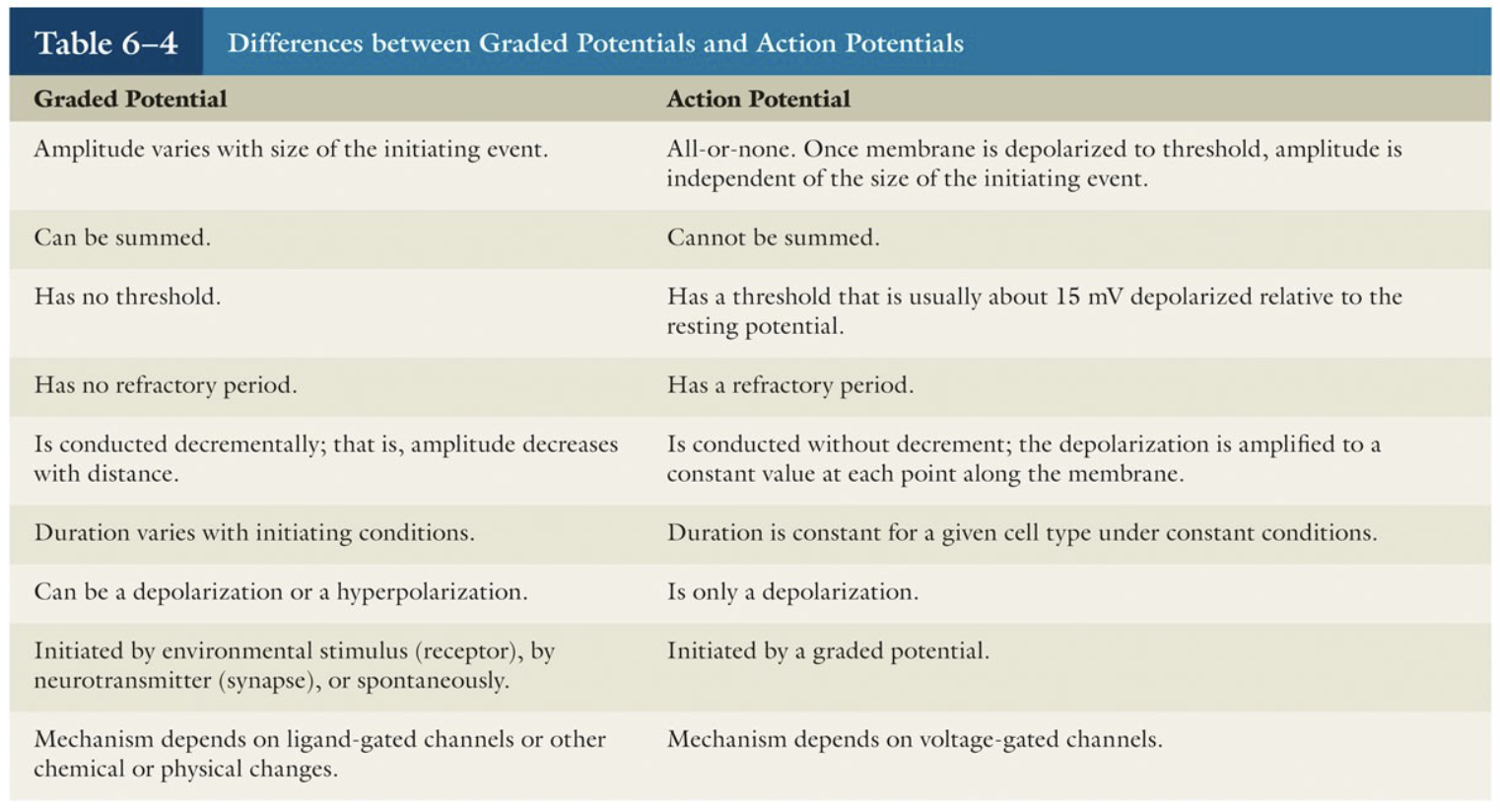
Graded Potentials
Short-lived, local (short distance signals
Stronger stimulus → increased magnitude
Spreads but current decays with distance, time (decremental)
Depolarizing or hyperpolarizing
Receptor potential
receptors of sensory neurons
Postsynaptic potential graded potential
dendrite or soma of neuron or muscle cell
Action Potentials
Principal means of long-distance neural communication
Only occur in muscle cells and axons of neurons (excitable cells)
Involves opening of specific voltage-gated channels
Brief reversal of membrane potential with a change in voltage of ~100 mV
Do not decay with distance
Voltage-gated Na+ and K+ channels give neurons ability to ____________
Generate and propagate action potentials
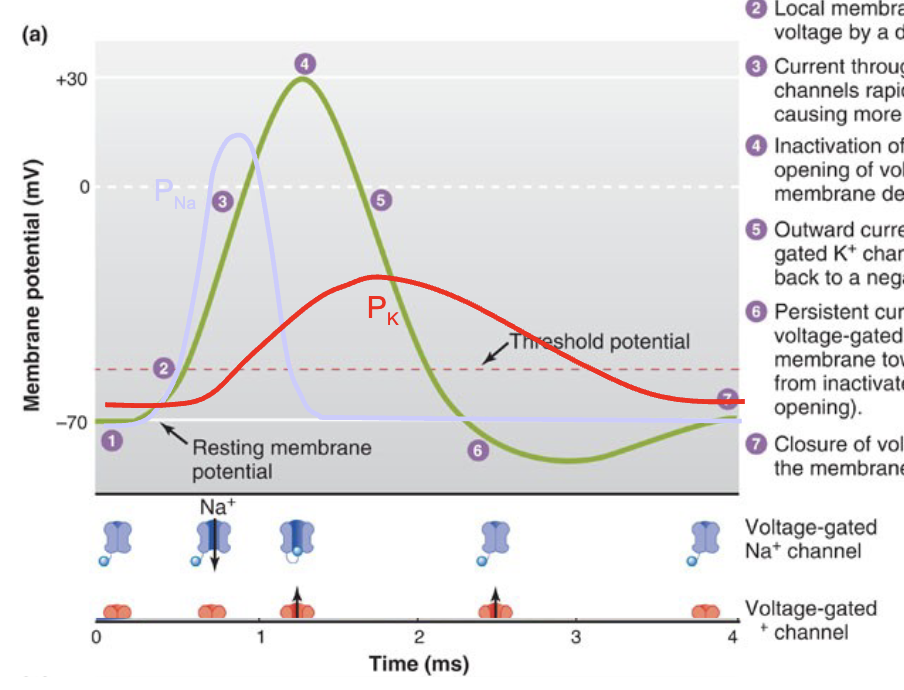
Action potential Mechanism
Steady resting membrane potential is near EK, PK>PNa, due to leak Kt channels.
Local membrane is brought to threshold voltage by a depolarizing stimulus.
Current through opening voltage-gated Na+ channels rapidly depolarizes the membrane, causing more Na* channels to open.
Inactivation of Nat channels and delayed opening of voltage-gated Kt channels halts membrane depolarization.
Outward current through open voltage-gated K* channels repolarizes the membrane back to a negative potential.
Persistent current through slowly closing voltage-gated K* channels hyperpolarizes membrane toward Ek; Na* channels return from inactivated state to closed state(without opening).
Closure of voltage-gated K* channels returns the membrane potential to its resting value.
Absolute refractory period
The brief interval following an action potential during which a neuron is completely unable to fire a second action potential, regardless of the stimulus strength
Relative refractory period
The phase following the absolute refractory period during which a neuron can fire a second action potential, but only in response to a stronger-than-normal stimulus
Threshold Potential
Depolarization triggers an AP only when the membrane potential exceeds a threshold potential (~-55 mV) .
Regardless of the size of the initial stimulus, if the membrane reaches threshold, the APs generated are all the same size and do not decay.
A single AP cannot convey information about the magnitude of the initial stimulus
Action Potential Thresholds
An action potential is an all-or-none event generated by a positive- feedback loop.
The threshold indicates whether or not incoming stimuli are sufficient to generate an action potential (i.e. whether the feedback loop will work).
The value of threshold can vary according to numerous factors.
Changes in the ion conductances of sodium or potassium, or the availability of voltage-gated Na+ channels, can lead to a raised or lowered value of threshold
Action Potential Propagation
Current entering during an AP is sufficient to easily depolarize adjacent membrane to the threshold potential.
Propagation of AP from the initial segment to the axon terminal is typically one-way because the absolute refractory period follows along in the “wake” of the moving AP
Action Potential Conduction Speed
Depends on fiber diameter and whether the fiber is myelinated.
Larger caliber axons allow current to flow more easily, speeding up propagation
Saltatory Conduction
The process where action potentials "jump" from one node of Ranvier to the next along a myelinated axon, significantly increasing the speed of nerve impulse transmission compared to unmyelinated axons
Graded Potentials vs Action Potentials
Synapse
Point of communication between two neurons (or neuron and muscle cell)
Chemical synapse
Neurotransmitters relay information from pre- to postsynaptic cell across synaptic cleft
Electrical synapse
pre- and post- synaptic cells joined by gap junctions
in CNS
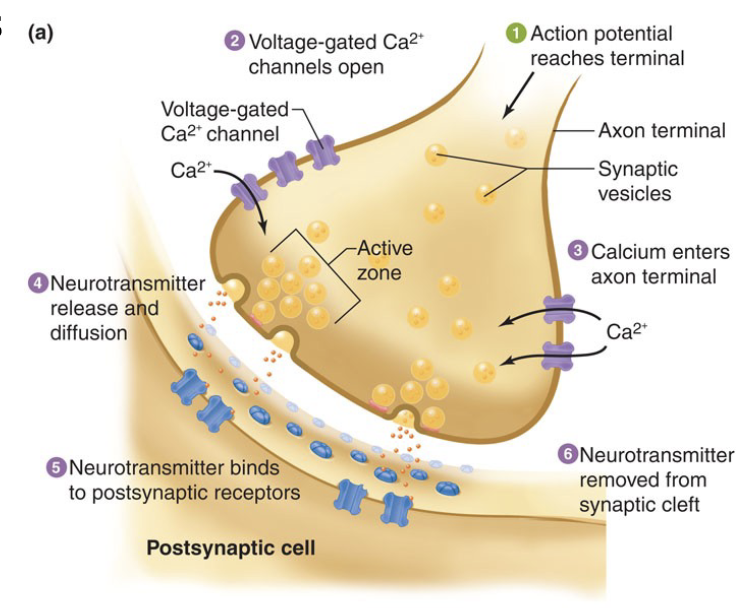
Mechanism of Neurotransmitter Release
Neurotransmitter stored in vesicles
Vesicles docked on PM at release regions known as active zones
Transmitter release initiated when AP reaches synaptic bouton
The AP depolarizes the membrane in bouton causing voltage-gated Ca2+ channels to open.
Ca2+ activates vesicle docking and fusion with the PM
Action. potential reaches terminal
Voltage-gates Ca2+ channels open
Calcium enters axon terminal
Neurotransmitter release and diffusion
Neurotransmitter binds to postsynaptic receptors
Neurotransmitter removed from synaptic cleft
Activation of the Postsynaptic Cell
NT diffuses across synaptic cleft (0.2 msec delay) and binds to postsynaptic receptors.
Ionotropic receptors – ligand-gated ion channels
Metabotropic receptors – mediate slower actions through G-protein second messengers
Excitatory Chemical Synapses
Glutamate
iGluRs: non-selective cation channels
EPSP: membrane potential closer to threshold
Changes in synaptic strength underlie learning and memory
Dendritic Spines
Protrude from the main shaft of dendrite, single synapse at head.
Heterogeneous in size, shape
Modified by activity and experience
Morphological basis for synaptic plasticity
Receptors, signaling proteins clustered in PSD
Inhibitory Chemical Synapses
GABA and glycine
Hyperpolarization caused by:
Opening Cl- channels in neurons where ECl < Er
Increased K+ permeability moves membrane potential towards EK (-90 mV)
IPSP causes what?
hyperpolarization (membrane potential further from threshold)
Synaptic Integration
Neurons undergo many EPSPs (A) and IPSPs (B)
Receive excitatory and inhibitory input
Axon Initial Segment
Lower threshold than axon for AP generation due to high VGSC concentration
Sensitive to small changes in the membrane potential that occur in response to synaptic potentials on soma and dendrites.
Location of individual synapses on the postsynaptic cell is important
Presynaptic modulation of synaptic strength
↑ [Ca2+i] during high frequency stimulation → increases NT release
Axo-axonic input (facilitation or inhibition)
Auto-receptors: -ve feedback decreases NT release
Postsynaptic
Paired-pulse facilitation
Desensitization – receptor responds then fails despite continued presence of NT (receptor internalization).
Drug effects on synaptic effectiveness
Release and degradation of the NT inside the axon terminal.
↑ NT release into the synapse.
↓ NT release into the synapse.
Inhibition of synthesis of the NT.
↓ reuptake of the NT from the synapse (e.g. Selective serotonin reuptake inhibitors (SSRIs) are antidepressants that affect serotonin levels in the brain)
↓ degradation of the NT in the synapse.
Agonists (evoke same response as neurotransmitter) or antagonists (block response to neurotransmitter) can occupy the receptors.
↓ biochemical response inside the dendrite
Subthreshold stimulus
doesn’t cause reaction
Threshold stimulus
Cause reaction
Single AP doesn’t tell us anything about _____
Strength of stimulus
Increase amplitude of stimulus=
Increased frequency of AP
Ionotropic receptors
Directly control ion channels and produce fast, brief responses
Metabotropic receptors
Use G proteins and second messengers for slower, more widespread, and long-lasting cellular effects