BIOL 3301 Chapter 19 Gene Mutation and DNA Repair, and Recombination
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
mutation
a heritable change in the genetic material
provide allelic variation
foundation for evolutionary change needed for a species to adapt to changes in the environment
new ? more likely to be harmful than beneficial to the individual and often are the cause of diseases
evolved
mutations can be quite harmful, therefore organisms have ? mechanisms to repair DNA damage
mutations
change in chromosome structure
change in chromosome number
changes in DNA of a single gene
can affect the molecular and phenotypic expression of genes
molecular: altered DNA sequence→ diff mRNA→ diff amino acid sequence→ altered phenotype
gene mutations
molecular changes in the DNA sequence of a gene
point mutation
a change in a single base pair
can involve a base substitution
transition: change pyrimidine→ pyrimidine or purine→ purine (more common)
transversion: pyrimidine→ purine or purine→ pyrimidine
transition
change pyrimidine→ pyrimidine
change purine→ purine
more common than the other type of base substitution
transversion
pyrimidine→ purine
purine→ pyrimidine
silent mutations
base substitutions that don’t alter the amino acid sequence of the polypeptide
due to degeneracy of the genetic code
missense mutations
those base substitutions in which an amino acid change does occur
ex., sickle cell disease
unlike sickle cell disease, a ? mutation may have no detectable effect on protein fx, , and the mutation is said to be neutral (more likely if new amino acid has similar chemistry to the amino acid it replaced)
neutral mutation
missesnse mutation may have no detectable effect n protein x. more likely to occur if new amino acid has chemistry to the amino acid it replaced
missense, nonsense, silent mutation
let’s say a transversion mutation occurred int he protein coding portion of a gene. what kind of mutation could this potentially cause?
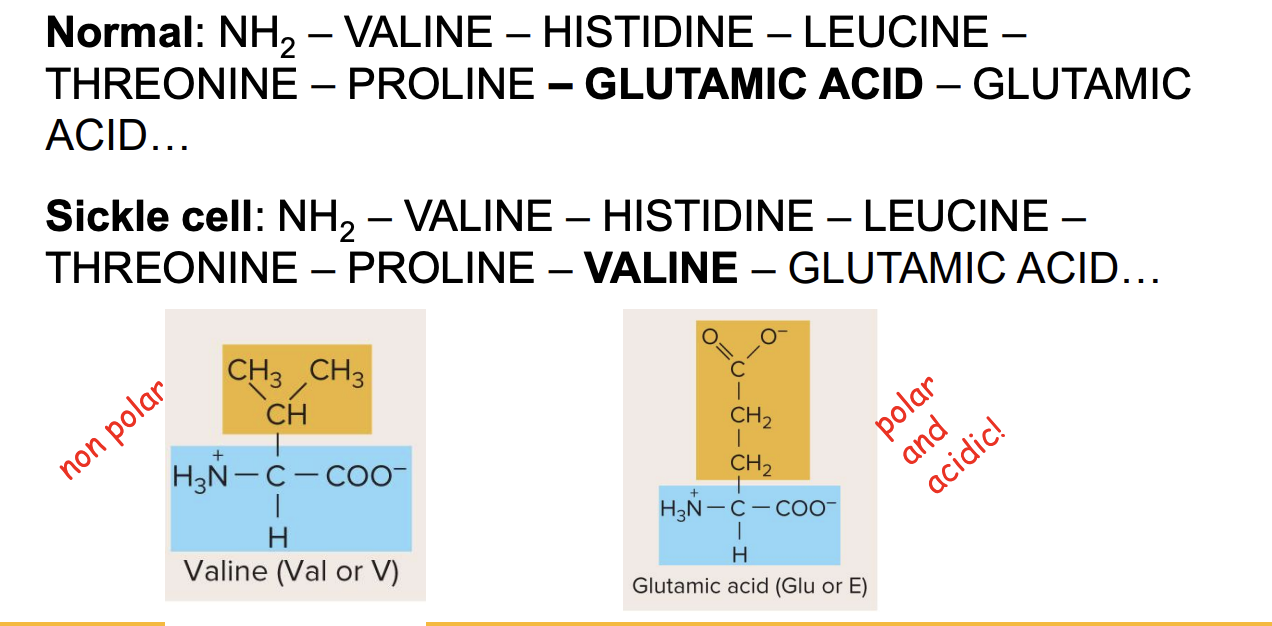
missense mutation in sickle cell
? mutation: changes a single amino acid in a protein
? caused by a ? mutation in the beta globin gene
mutation changes Glu to Val at position 6
mutation leads to abnormal Hb, causing RBCs to sickle and affect O2 transport
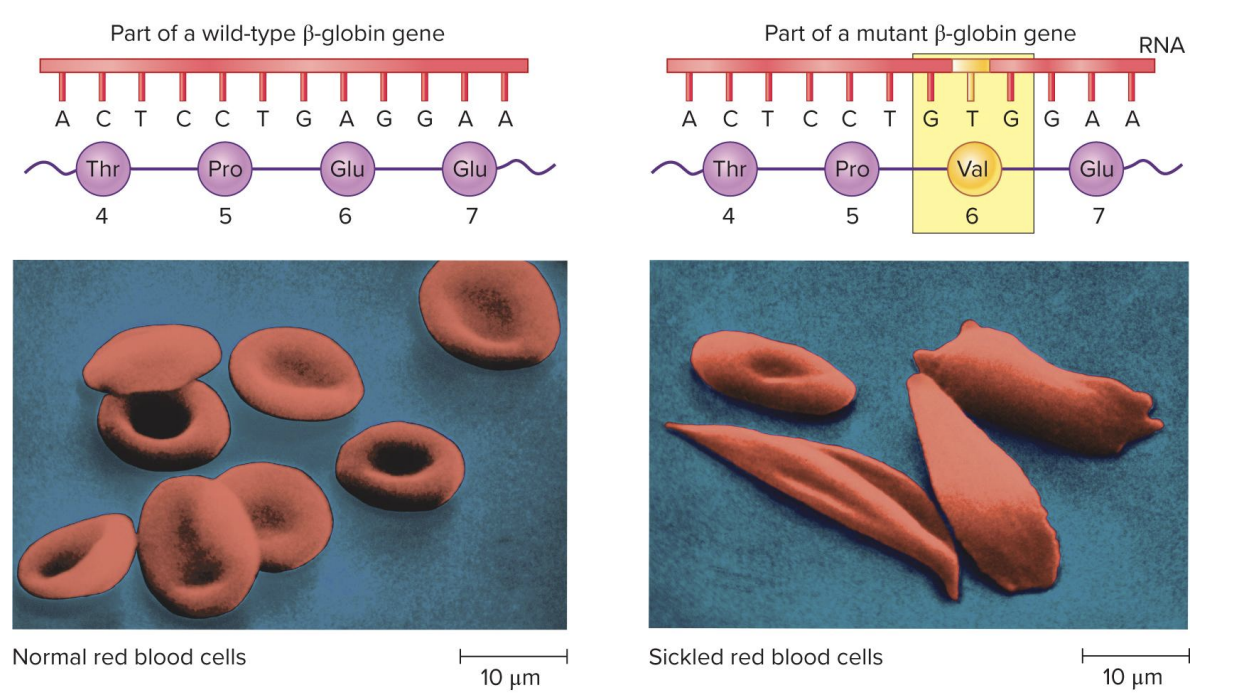
glutamic acid→ valine
missense mutation at position 6 of sickle cell disease
normal beta globin vs sickle cell beta globin
originally polar and acidic→ nonpolar
NH2- Valine- histidine- leucine-threonine-proline-?- glutamic acid
mutations
alter the coding sequence within a protein-encoding gene; can have various effects on polypeptide
nonsense: base substitutions that change a normal codon to a stop codon
frameshift: add/delete a number of nucleotides not divisible by 3
shifts the reading frame so that translation of the mRNA results in a completely different amino acid sequence downstream of the mutation
nonsense mutations
base substitutions that change a normal codon to a stop codon
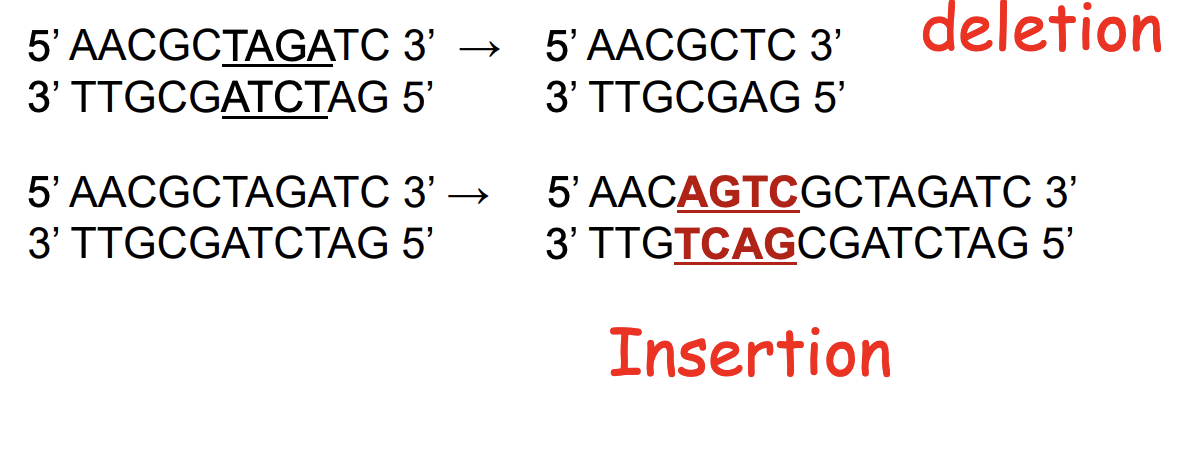
frameshift mutations
add/delete a number of nucleotides not divisible by 3
shifts the reading frame so that translation of the mRNA results in a completely different amino acid sequence downstream of the mutation
indels
mutations may also involve the addition or deletion of short sequences of DNA
silent mutation
base substitution
no amino acids altered
prob no effect on protein fx
mutated mRNA sequence is the same as the coding strand (but U instead of T). template strand is complementary and antiparallel to coding strand.
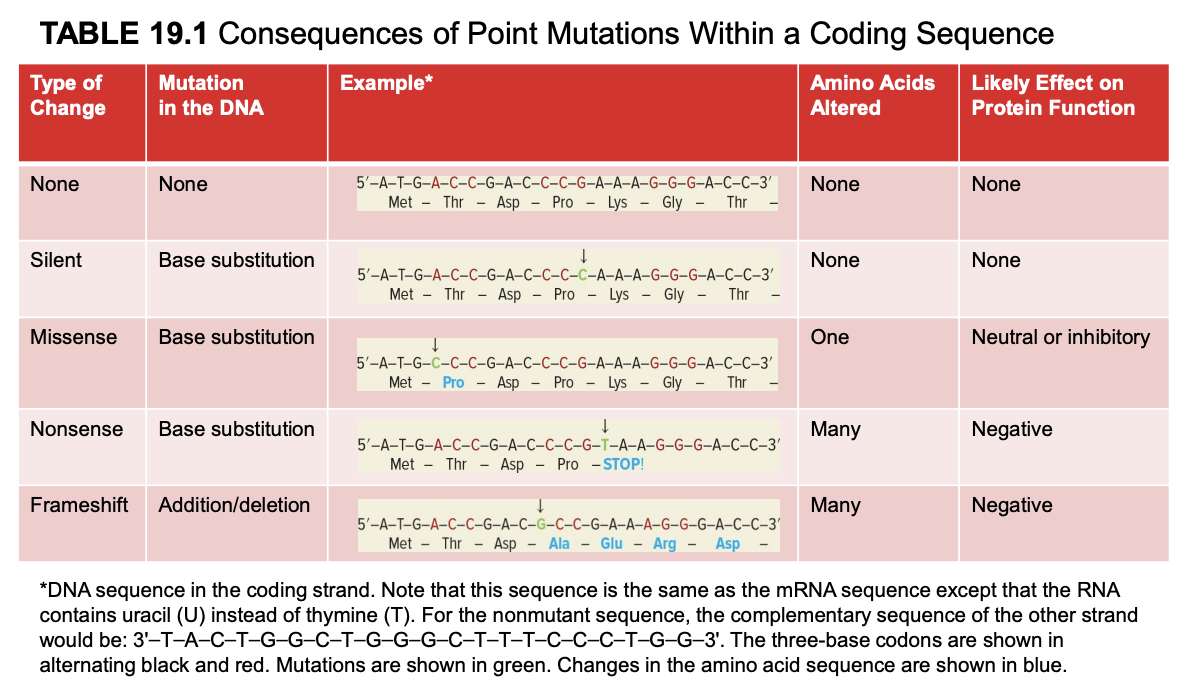
missense mutation
base substitution
1 amino acid altered
neutral or inhibitory effect on protein fx
mutated mRNA sequence is the same as the coding strand (but U instead of T). template strand is complementary and antiparallel to coding strand.
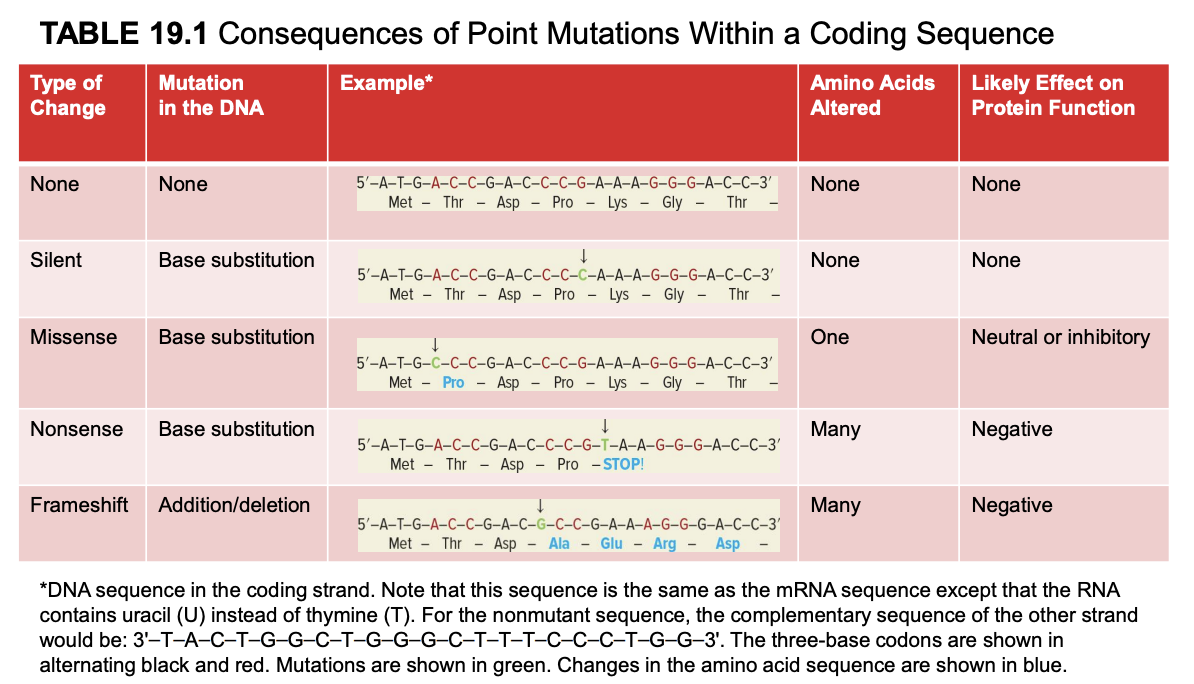
nonsense mutation
base substitution to change a normal codon→ stop codon
many amino acids altered
negative effect on protein fx
mutated mRNA sequence is the same as the coding strand (but U instead of T). template strand is complementary and antiparallel to coding strand.
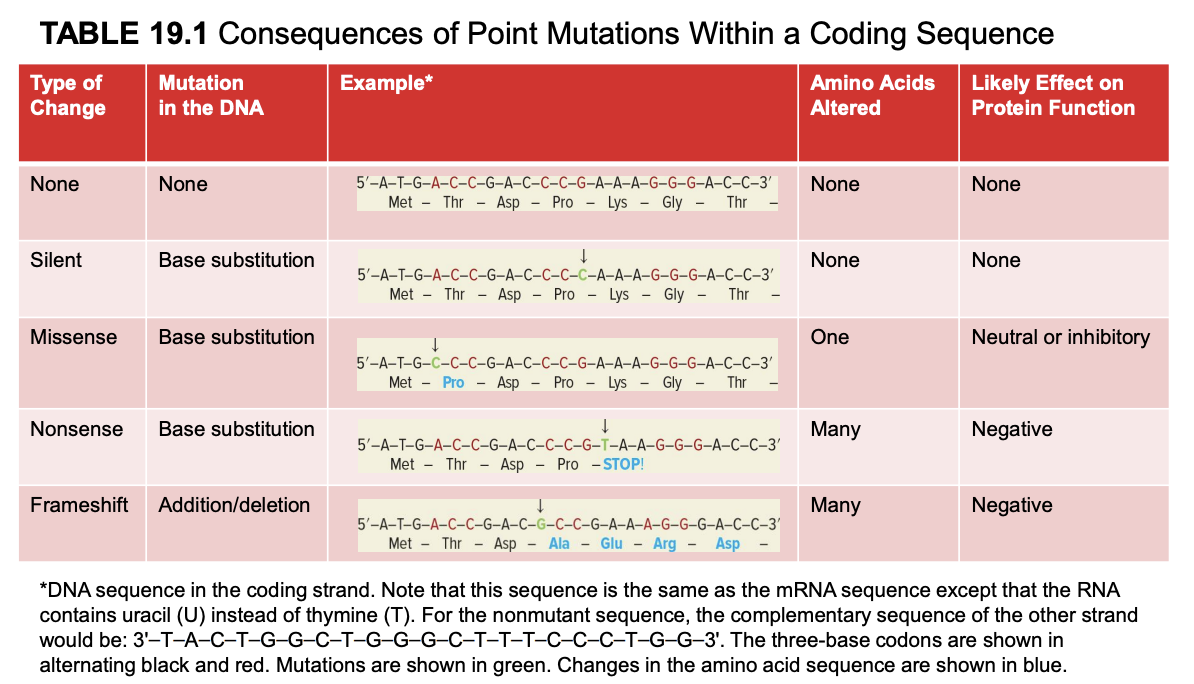
frameshift mutation
addition/deletion
many amino acids altered
negative effect on protein fx
mutated mRNA sequence is the same as the coding strand (but U instead of T). template strand is complementary and antiparallel to coding strand.
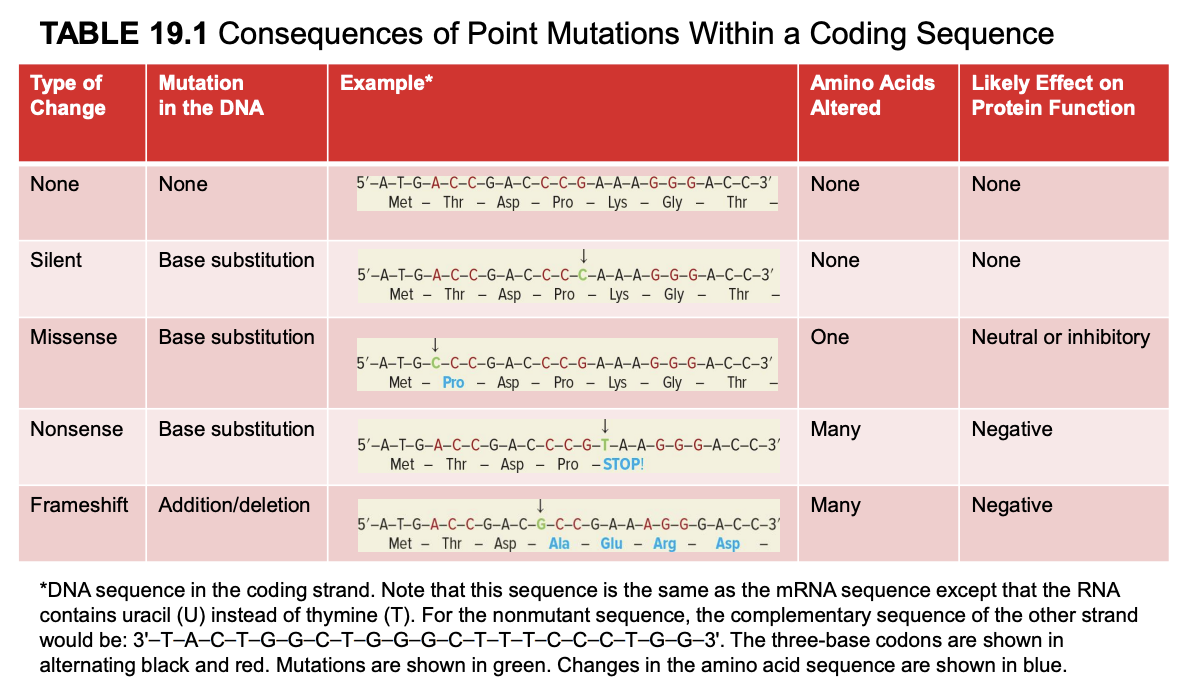
wildtype
the relatively prevalent genotype in a natural population
genes with multiple alleles may have 2 or more ?
forward mutation
changes the wild-type genotype into some new variation
reversion mutation
changes a mutant allele back to the wild-type
deleterious mutations
decrease the chances of survival
most extreme are lethal mutations
beneficial mutations
enhance the survival or reproductive success of an organism
environment
the ? can affect whether a given mutation is deleterious or beneficial
conditional
some mutations are ?
they affect phenotype only under a defined set of conditions
ex., temperature-sensitive mutation
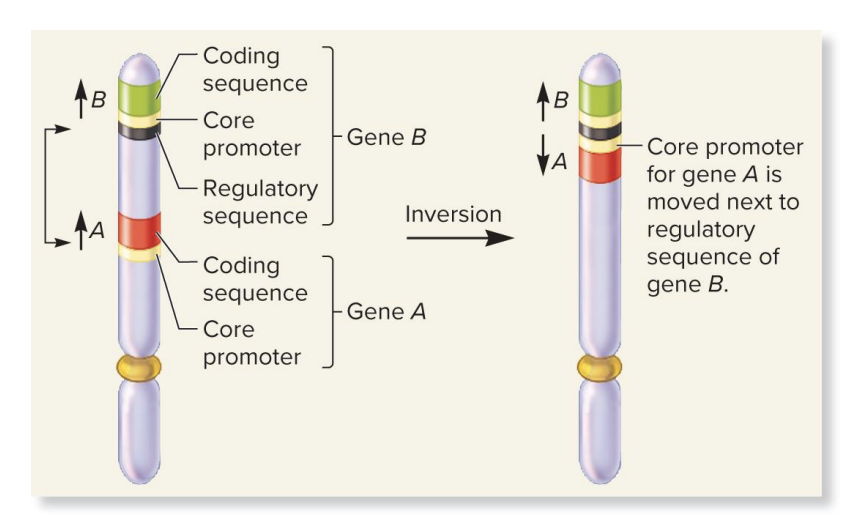
position effect due to regulatory sequences
a chromosomal inversion flips a section of DNA
this move’s gene A’s promoter next to gene B’s regulatory sequence
since regulatory sequences can work in both direction (bidirectional), gene B’s regulatory elements may now activate gene A
regulatory sequences are often bidirectional, so gene A may now show the expression pattern of gene B
gene A is now expressed abnormally
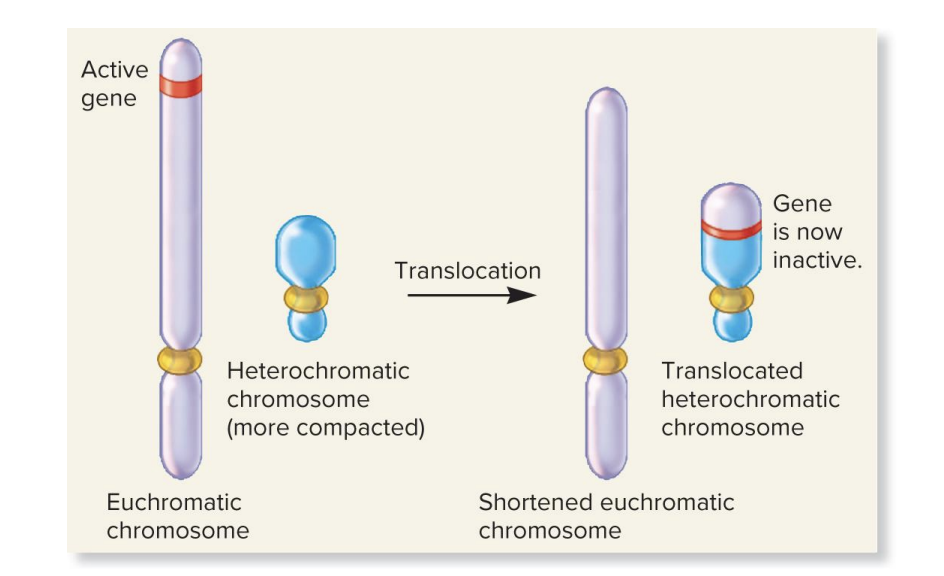
position effects from translocation to a heterochromatic chromosome
a ? moves a gene from a euchromatic (open, active) region to a heterochromatic (dense, silent) region
in heterochromatin (dense, silent) region
in heterochromatin, DNA is tightly packed and genes are turned off
effect: the relocated gene becomes
breakpoint
a chromosomal rearrangement may affect a gene bc the chromosomal ? occurs within the gene
site of breaking and rejoining
position effect
a gene may be left intact after a chromosomal rearrangement, but its expression may be altered bc of its new location
2 reasons
movement to a position next to regulatory sequences
gene A may show expression pattern of gene B
movement (translocation) to a heterochromatic region (which is a condensed chromatin and not expressed)
gene becomes inactive, even though its sequence is unchanged bc the gene is moved into a silenced region of the genome
germ line cells
cells that give rise to gametes such as eggs and sperm
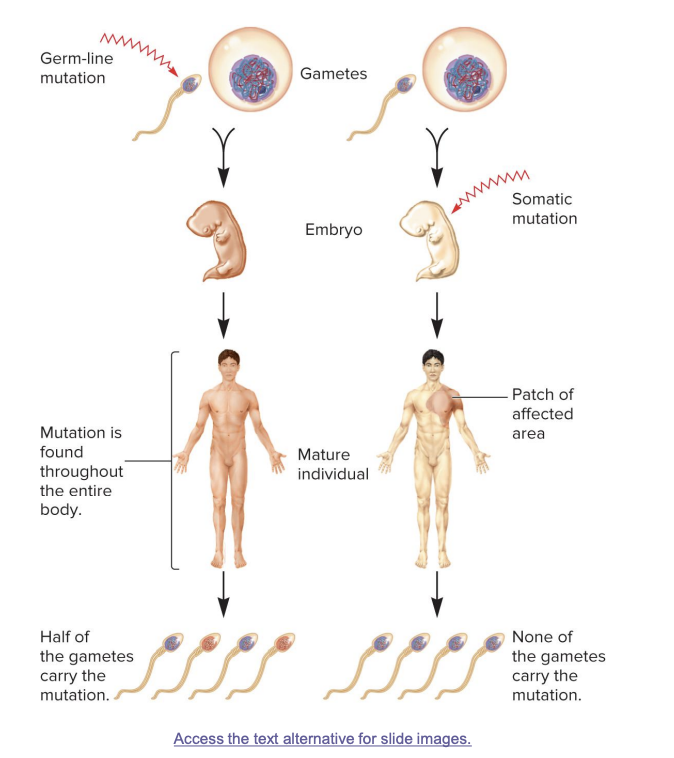
somatic cells
all other non-egg/sperm cells
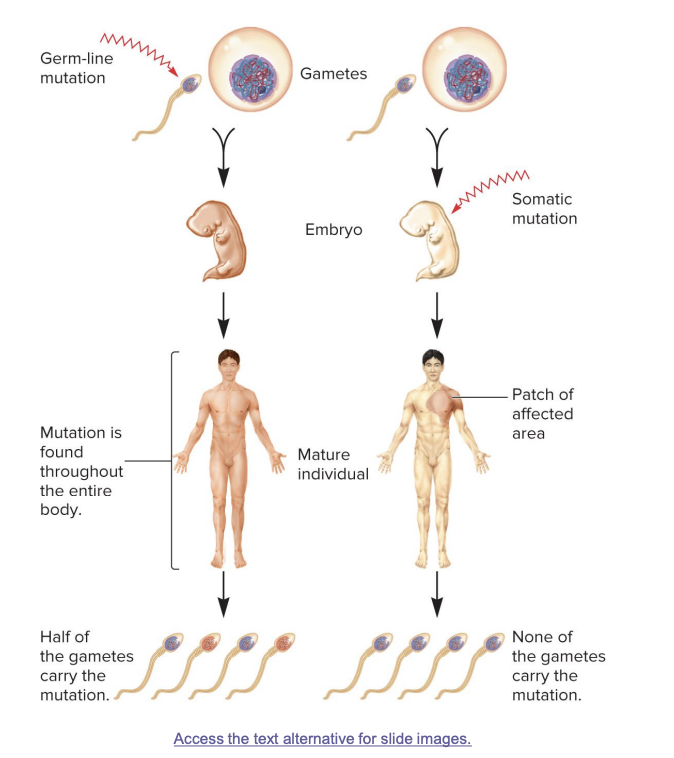
germ line mutations
occur directly in a sperm or egg cell, or in one of their precursor cells
passed on to offspring
mutation is found in every cell of the body
half of the gametes in the mature individual may carry the mutation
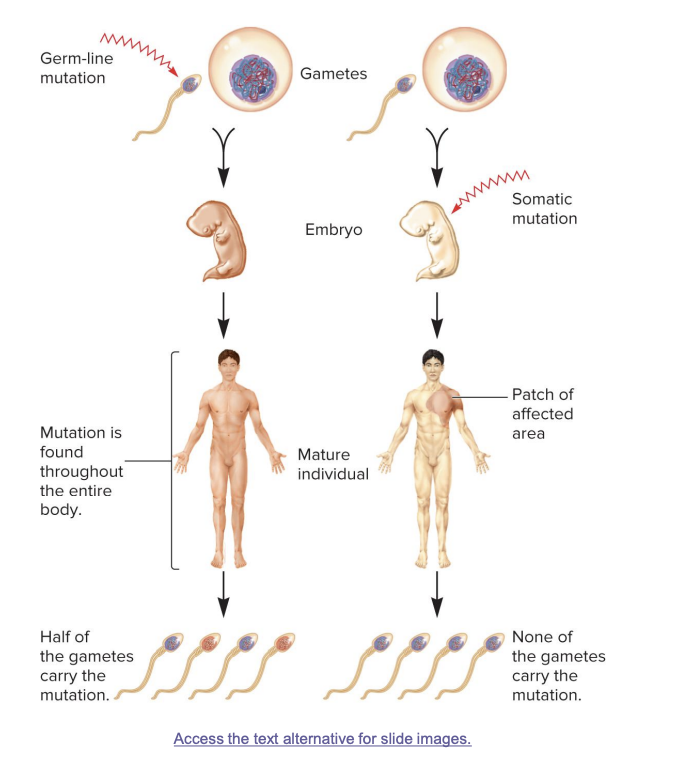
somatic mutations
occur directly in a body cell that is not part of the germ-line
occurs in body cells after fertilization
not inherited; affects only parts of the body (a “patch”)
mutation is found in a specific area of the body
no gametes carry the mutation
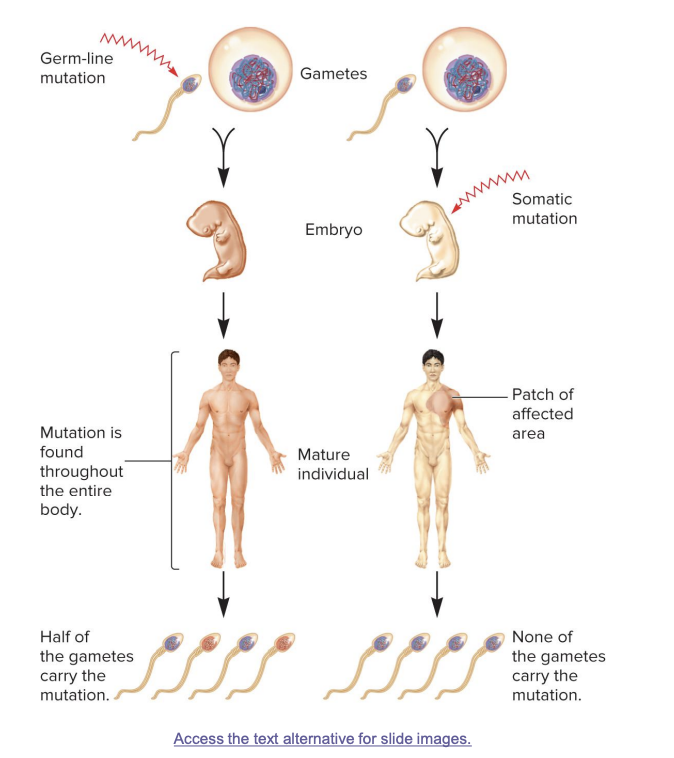
germ line mutations
occur in gametes
passed on to half of the gametes in the next generation; mutation found in whole body
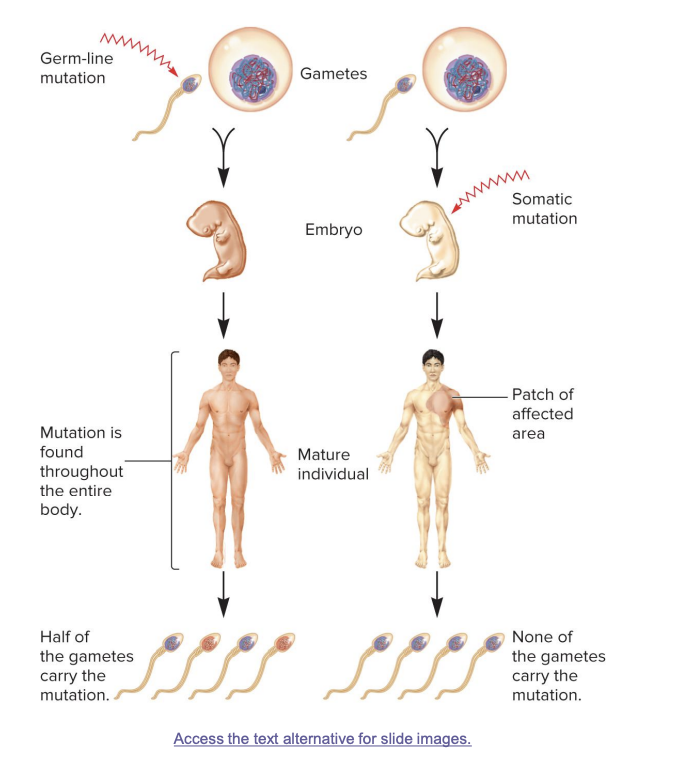
somatic mutations
result in patches of affected area
the size of the patch will depend on the timing of the mutation. the earlier the mutation, the larger the patch
an individual with ? regions that are genotypically different from the rest of the body is called a genetic mosaic
mutations not present in gametes
genetic mosaic
an individual with somatic regions that are genotypically different from the rest of the body
spontaneous mutations
result from abnormalities in cellular/biological processes
e.g., errors in DNA replication
underlying cause originates within the cell
induced mutations
caused by environmental agents
agents that are known to alter DNA structure are termed mutagens
these can be chemical or physical agents
spontaneous deamination of cytosine
removal of an amino group from the cytosine base
the other bases are not readily deaminated
converts cytosine→ uracil + NH3
uracil is not normally found in DNA, so this can be recognized and repaired
DNA repair enzymes can recognize uracil as an inappropriate base in DNA and remove it
however, if the repair system fails, a C-G to A-T mutation will result during subsequent rounds of DNA replication
C-G base pair becomes an A-T base pair → permanent mutation
spontaneous deamination of 5 methylcytosine
5 methylcytosine can be deaminated into thymine, a normal constituent of DNA
5 methylcytosine→ thymine + NH3
thymine is a normal DNA base, so this mutation is harder to detect and repair may lead to permanent base changes
repair enzymes cannot determine which of the 2 bases on the 2 DNA strands is the incorrect base
for this reason, methylated cytosine bases tend to create hot spots for mutation
oxidative stress
an imbalance btw the production of reactive oxygen species (ROS) and an organisms’s ability to break them down
ROS: hydrogen peroxide, superoxide, hydroxyl radical
may lead to DNA damage and mutation
ROS over accumulation can lead to oxidative DNA damage
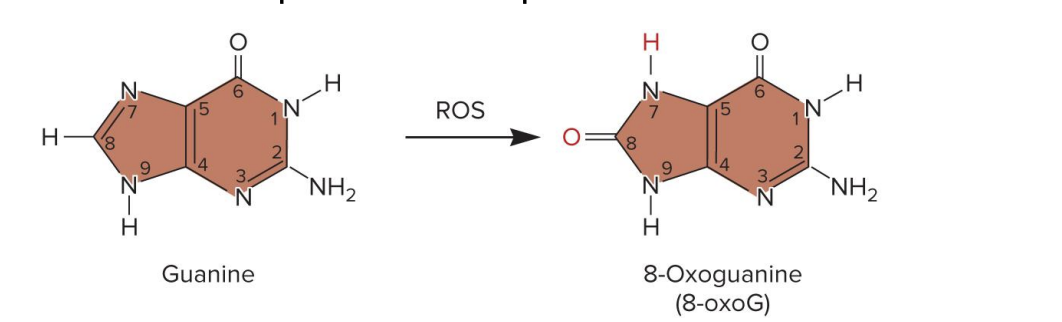
ex., guanine → 7, 8-dihydro 7-oxoguanine (8-oxoG)
8-oxoG mistakenly pairs with adenine during replication (normal G pairs with C)
the original G-C base pair becomes a T-A base pair→ permanent point mutation
reactive oxygen species ROS
aerobic organisms produce ? which include
hydrogen peroxide
superoxide
hydroxyl radical
body tries to block this buildup (bc accumulation can lead to oxidative DNA damage)
enzymes
superoxide dismutase and catalase
antioxidants
oxidative DNA damage
results from reactive oxygen species ROS overaccumulation
hydrogen peroxide, superoxide, hydroxyl radical
body enzymes like superoxide dismutase and catalase, and antioxidants don’t break enough ROS down
ex., guanine → 7, 8-dihydro 7-oxoguanine (8-oxoG)
8-oxoG mistakenly pairs with adenine during replication (normal G pairs with C)
the original G-C base pair becomes a T-A base pair→ permanent point mutation
trinucleotide repeat expansion
several human genetic diseases are caused by an unusual form of mutation
e.g., huntington’s disease (autosomal dominant, lethal, no cure)
certain regions of the chromosome have short sequences repeated in tandem
in unaffected individuals, these repeats are stable and passed on wo mutation
in affected individuals, the length of? has increased above a certain critical size
disease symptoms occur
in some cases, the expansion is within the coding sequence of the gene
typically, the ? is CAG (glutamine)
therefore, the coded protein will contain long tracks of glutamine
this causes the proteins to aggregate with each other
this aggregation is correlated with the progression of the disease, but may not cause disease symptoms
some ? disorders progressively worsen in future generations
may depends on which parent the mutant allele comes from
in huntington disease, the ? is more likely to occur if inherited from the male parent
in myotonic muscular dystrophy, the ? is more likely to occur if inherited from the female parent
suggests that ? can occur more frequently during oogenesis or spermatogenesis, depending on the gene involved
these changes can occur during gamete formation
offspring will have very different numbers of repeats
can also increase in somatic cells as a person ages
this can increase severity of the disease over time
huntingtons’s disease
trinucleotide repeat more likely to expand if inherited from the father
suggests TRNEs can occur more frequently during oogenesis or spermatogenesis, depending on the gene involved
myotonic muscular dystrophy
trinucleotide repeat more likely to expand if inherited from the mother
suggests TRNEs can occur more frequently during oogenesis or spermatogenesis, depending on the gene involved
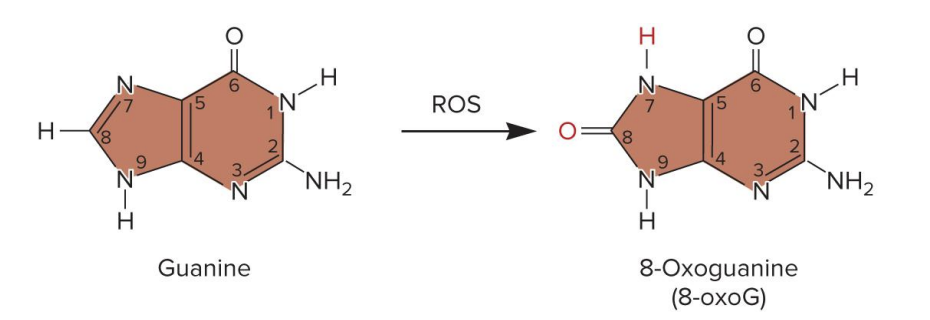
trinucleotide repeat expansion TRNE mechanism
? can expand during DNA replication
trinucleotide repeat sequences (e.g., CTG, CAG) are present in the DNA
these repeats can form hairpin loops due to C-G base pairing
during DNA replication
DNA polymerase replicates thru the repeat region
a hairpin forms in the new (daughter) strand
hairpin causes DNA polymerase to slip off
when DNA polymerase resumes replication, it recopies the repeat region
this results in a longer repeat region in the daughter strand
DNA gap repair seals the new strand, locking in the extra repeats
TRNE expands, over time this leads to disease-causing mutations
mutagens
agents that alter the DNA structure and thereby cause mutations
type of induced mutations
2 primary concerns
? often involved in the development of human cancers
? can cause gene mutations that may have harmful effects in future generations
an enormous array of agents can act as ?
usually classified as chemical or physical
chemical mutagens
3 main types
base modifiers
some covalently modify base structure
others disrupt pairing by alkylating bases
intercalating agents
directly interfere with replication process
slip btw DNA base pairs, distorting DNA structure, which interferes with DNA replication
can cause insertions or deletions
base analogues
incorporate into DNA and disrupt structure and normal base pairing
look like normal bases, but aren’t. can be mistakenly used by cell and inserted into DNA during replication
mutagens
agents that alter the DNA structure and thereby cause mutations
type of induced mutations
2 primary concerns
? often involved in the development of human cancers
? can cause gene mutations that may have harmful effects in future generations
tldr
chemical that react with bases change their structure and make them pair weird
physical mutagens
often cause breaks or abnormal bonds in DNA
include radiation
X rays, gamma rays, ionizing radiation, UV light
agents that alter the DNA structure and thereby cause mutations
type of induced mutations
2 primary concerns
? often involved in the development of human cancers
? can cause gene mutations that may have harmful effects in future generations
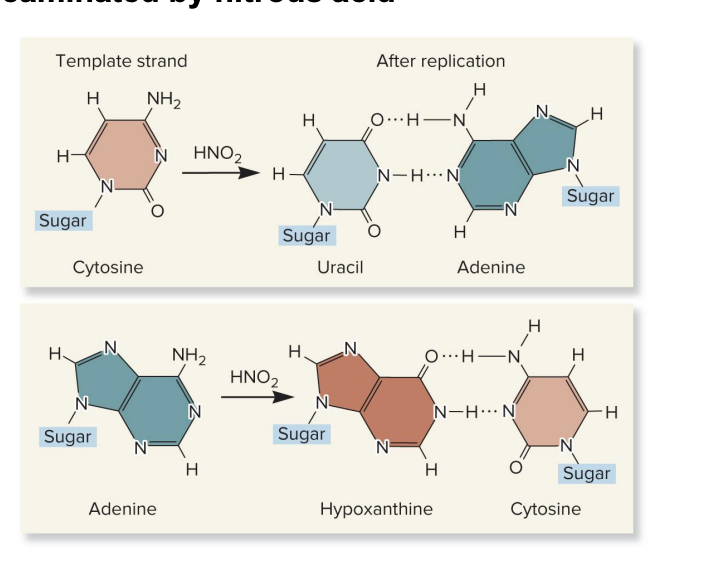
nitrous acid
a type of CHEMICAL MUTAGEN
chemically reacts with DNA bases
causes deamination (removal of an amino group) of:
cytosine→ uracil
U pairs with A during replication
this causes a C-G to a T-A mutation over time
adenine→ hypoxanthine
hypoxanthine pairs w/ cytosine
this causes an A-T to G-C mutation over time
base deamination by ? leads to incorrect base pairing, which results in point mutations after DNA replication
base analogues
become incorporated into daughter strands during DNA replication
look like normal bases, but aren’t
ex., 5 bromouracil is a thymine analogue
it can be incorporated into DNA instead of thymine
it can pair with guanine or adenine
keto form→ pairs with adenine (normal)
enol form→ pairs with guanine (abnormal)
chemical mutagen that increase the chance of transition mutations (purine-purine or pyrimidine-pyrimidine switch)
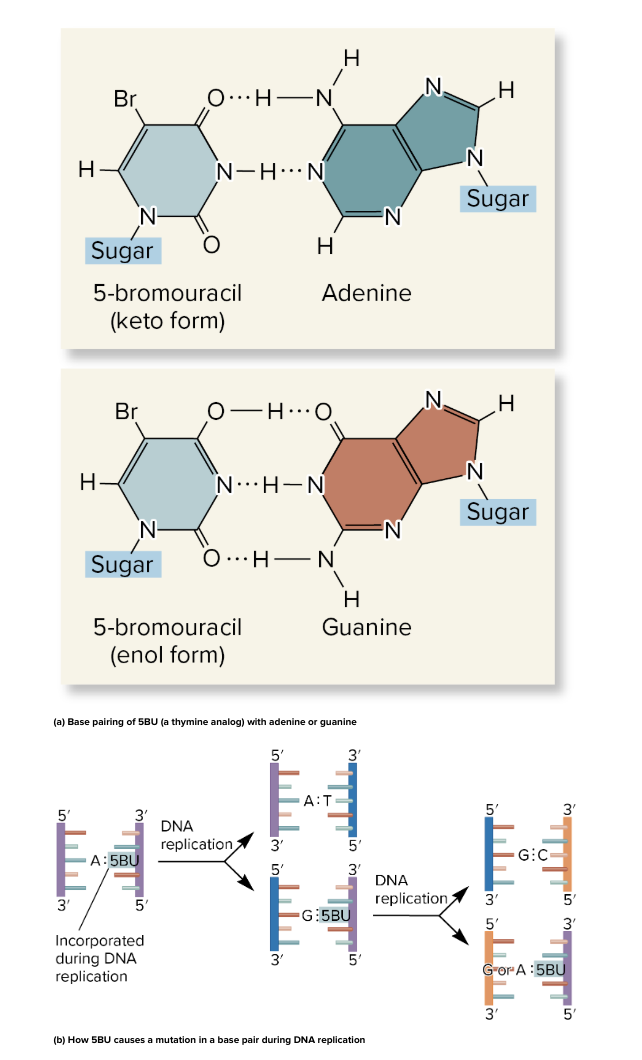
5 bromouracil 5BU
5BU is a base analogue (looks like thymine)
can exists in 2 forms
keto form→ pairs with A (normal)
enol form→ pairs with G (abnormal)
5BU incorporated into DNA instead of thymine
if 5BU switches to enol form, it mispairs with G
after 1 round of replication, G is in place of A
after another round, a G-C pair replaces original A-T
result: a permanent point mutation (A-T → G-C)
a chemical mutagen that increases the chance of transition mutations (purine-purine or pyrimidine-pyrimidine switch)
ionizing radiation
a type of physical mutagen
ex., X rays, gamma rays
short wavelength
high energy
can penetrate deeply into biological molecules
creates chemically reactive molecules (FREE RADICALS)
can cause
base deletions
oxidized bases
single nicks in DNA strands
cross linking
chromosomal breaks
nonionizing radiation
type of physical mutagen
ex., UV light
has less energy
cannot penetrate deeply into biological molecules
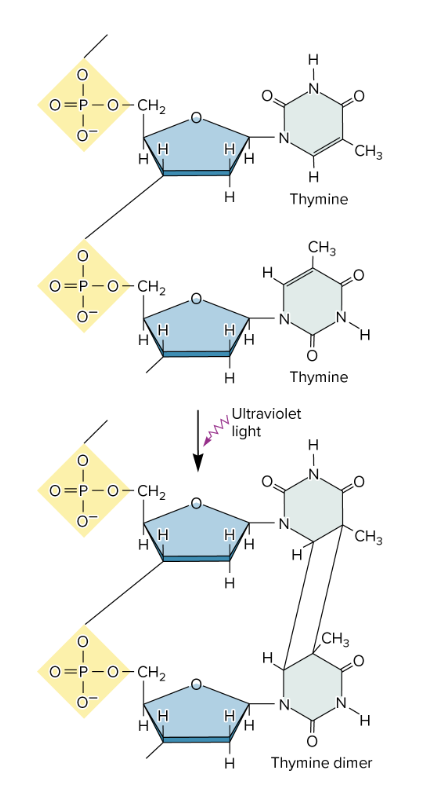
causes the formation of cross-linked thymine dimers
thymine dimers form when UV light hits DNA
2 adjacent thymine bases on same DNA strand that covalently bond> distort DNA double helix (bulky, abnormal structure)
thymine dimers may cause mutations when that DNA strand is replicated
if not repaired by nucleotide excision repair
mutation rate
the likelihood that a gene will be altered by a new mutation
commonly expressed as the # of new mutations in a given gene per cell generation
how likely a gene is to be altered by a new mutation.
number of new mutations per cell generation.
range of 10^-5 to 10^-9 per generation
humans: each generation adds about 100-200 new mutations to the genome (100-200 mutations/generation)
? for a given gene isn’t constant
can be increased by presence of mutagens
vary substantially btw species and even within different strains of the same species
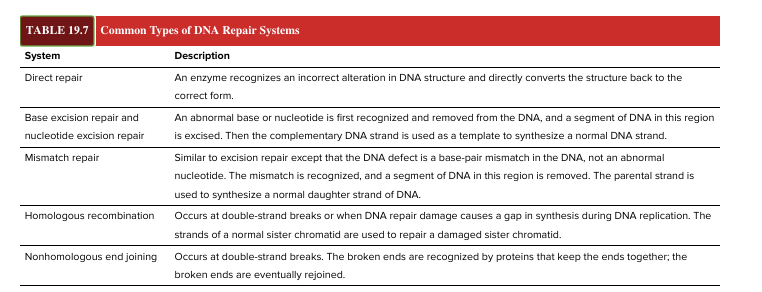
DNA repair
vital to the survival of all organisms bc most mutations are deleterious
living cells contain several ? systems that can fix different type of DNA alterations
direct repair
base excision repair and nucleotide excision repair
mismatch repair
homologous recombination
nonhomologous end joining (for double stranded breaks!)
in most cases, ? is a multistep process
an irregularity in DNA structure is detected
the abnormal DNA is removed
normal DNA is synthesized
direct repair
enzyme recognizes an incorrect alteration in DNA structure and directly converts the structure back to the correct form
specific enzymes can reverse the covalent modifications of nucleotides
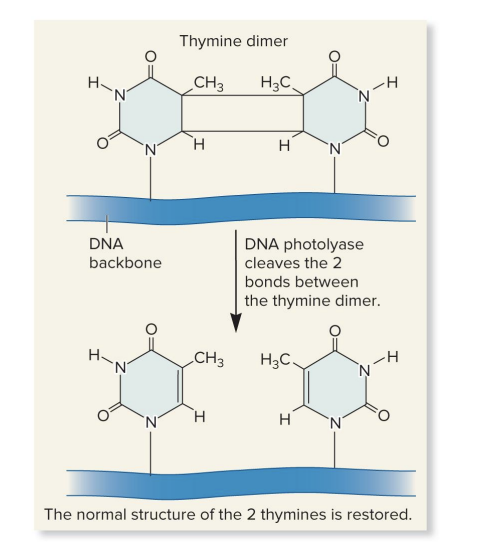
photolyase
repair thymine dimers
splits the dimers restoring the DNA to its original condition
uses energy of visible light for photoreactivation
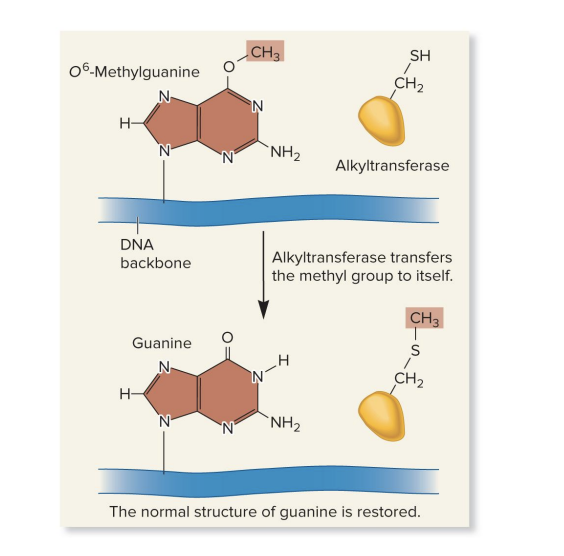
alkyltransferase
repairs alkylated bases
transfers the methyl or ethyl group from the base to a cysteine side chain within the alkyltranferase protein
surprisingly, this permanently inactivates alkyltransferase
photolyase
enzyme that performs direct repair of a thymine dimer
thymine dimers form when UV light causes 2 adjacent thymines to bond
uses visible light energy to break the bonds btw the thymines (photoreactivation)
splits the dimers
restore DNA to normal structure (2 separate thymines)
alkyltransferase
performs direct repair of an alkylated base
sometimes guanine is incorrectly modified into O⁶-methylguanine (a mutagenic, alkylated base)
repairs alkylated bases
removes the methyl (or ethyl) group from the damaged base
transfers the group to a cysteine side chain on itself
this repair action permanently inactivates the ? enzyme
the enzyme can only be used once (“suicide enzyme”)
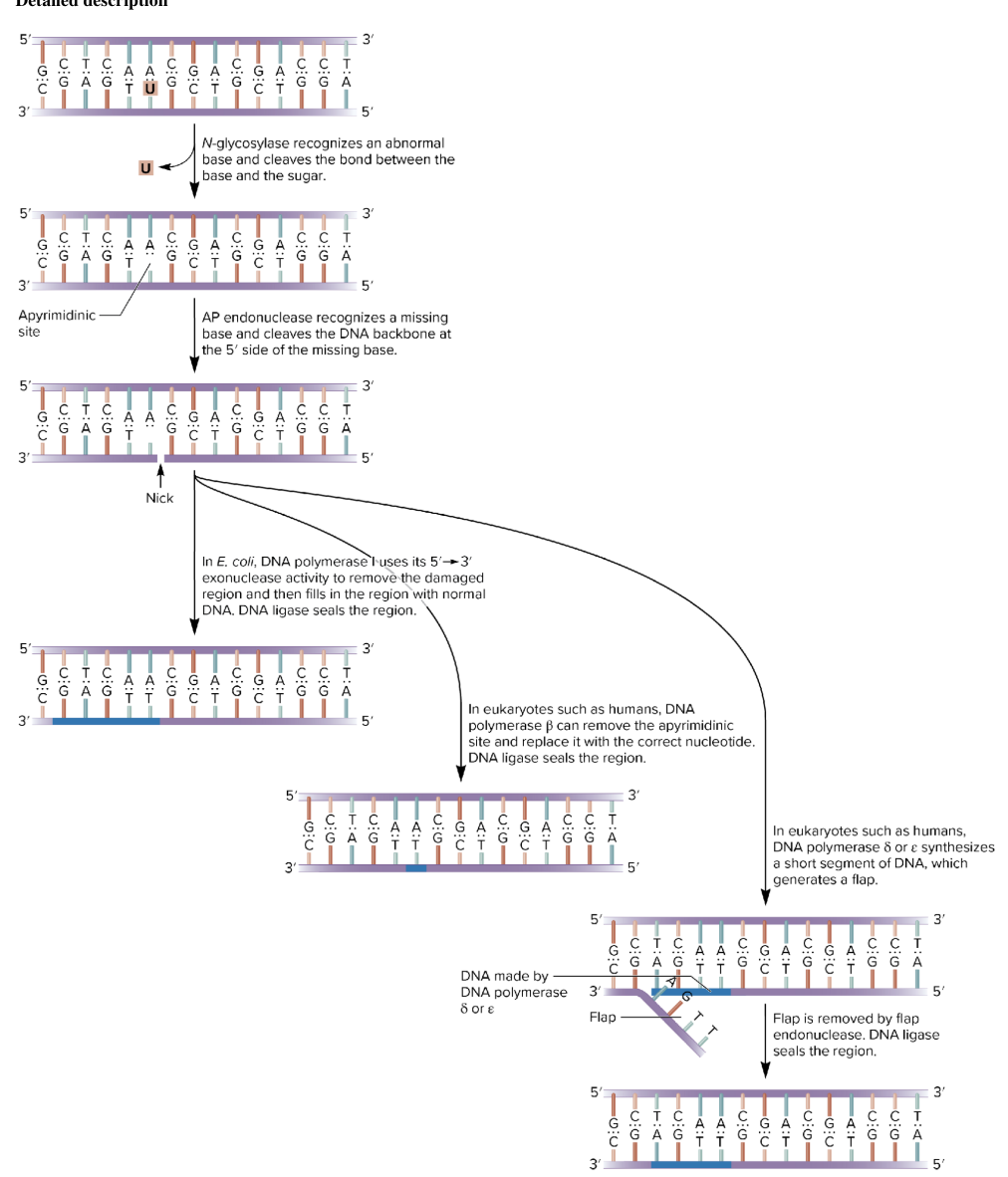
base excision repair BER
removes a damaged base, a segment of DNA in this region is excised, then the complementary DNA strand is used as a template to synthesize a normal DNA strand
involves DNA N-glycosylases enzyme category
recognize an abnormal base and cleave the bond btw it and the sugar in DNA
depending on the species, this repair system can eliminate abnormal bases like
uracil; 3-methyladenine; 7-methylguanine
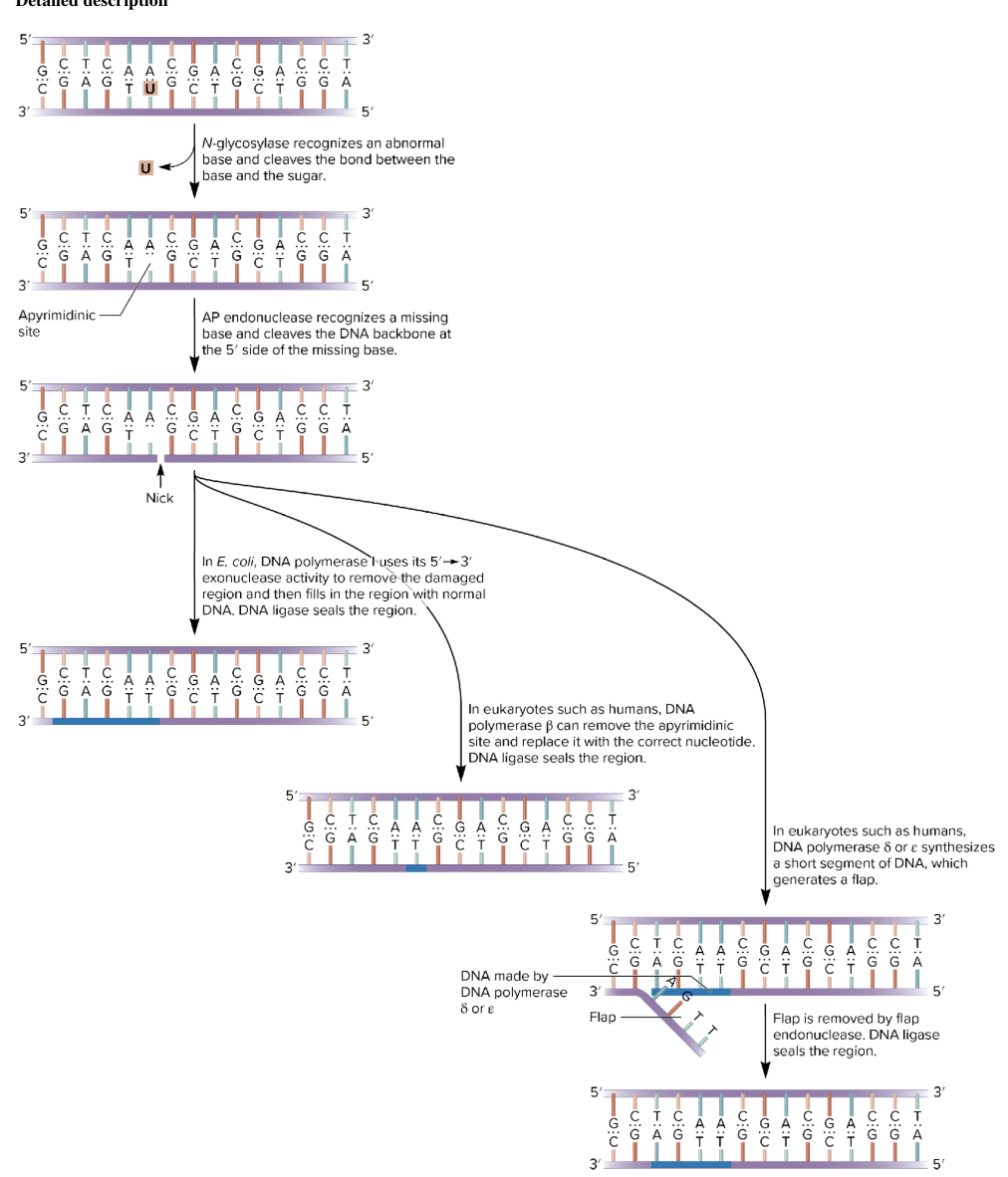
base excision repair BER
DNA N-glycosylases detects and removes the abnormal base
removes an abnormal base and cleaves the bond btw the base and the sugar
leaves an AP site (apurinic/apyrimidinic site) (missing purine or pyrimidine)
AP endonuclease cuts the DNA backbone at the 5’ side of the missing base
DNA polymerase
in E.coli:
DNA pol I 5’ → 3’ exonuclease removes damaged section and fills in correct nucleotides, DNA ligase seals the region
in humans
Pol β can remove and replace just one nucleotide. DNA ligase seals.
Or Pol δ/ε synthesize a new strand (flap formed).
flap is removed by flap endonuclease. DNA ligase seals the region
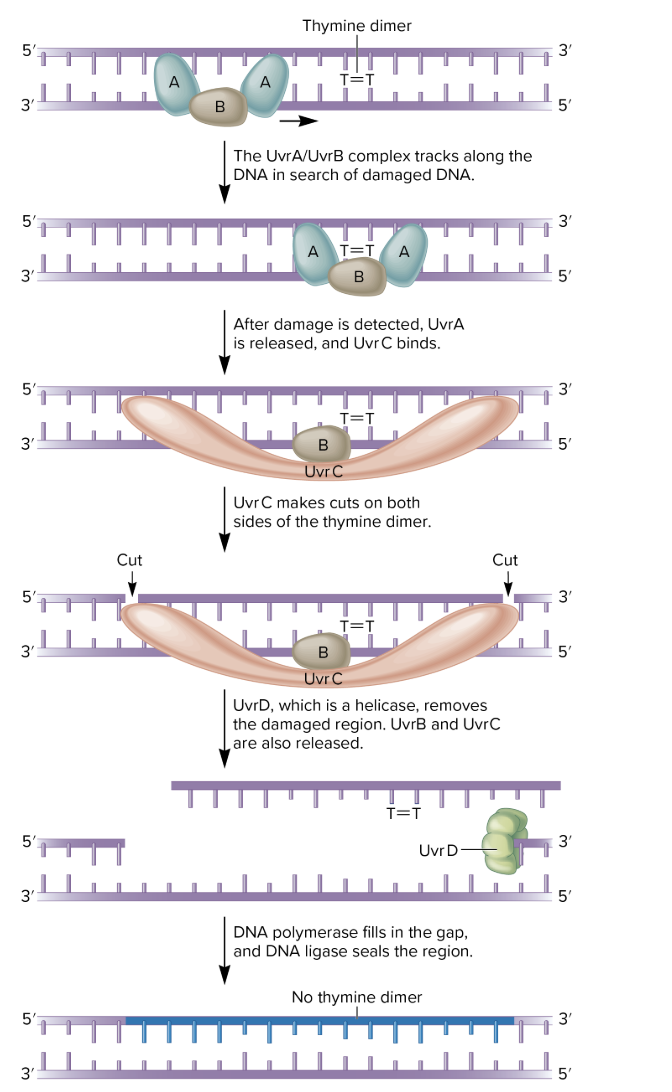
nucleotide excision repair NER
several nucleotides in the damaged strand are removed from the DNA and the undamaged strand is used as a template to resynthesize a normal strand
can repair many types of DNA damage
thymine dimers and chemically modified bases
missing bases
some types of crosslinks
found in all eukaryotes and prokaryotes
however, its molecular mechanism is better understood in prokaryotes
in E coli
4 proteins required
UvrA, UvrB, UvrC, UvrD
involve in UltraViolet light Repair of pyrimidine dimers
also important in repairing chemically damaged DNA
UvrA, B, C, and D recognize and remove a short segment of damaged DNA
DNA polymerase and ligase finish the repair job
in humans
several human diseases have been showed to involve inherited defects in genes involved in ?
xeroderma pigmentosum (XP)
can be caused by defects in 7 different ? genes
cockayne syndrome CS
increased sensitivity to sunlight is a common characteristic
nucleotide excision repair in ecoli
several nucleotides in the damaged strand are removed from the DNA and the undamaged strand is used as a template to resynthesize a normal strand
can repair many types of DNA damage
thymine dimers and chemically modified bases
missing bases
some types of crosslinks
proteins required
UvrA, UvrB, UvrC, UvrD
involve in UltraViolet light Repair of pyrimidine dimers
also important in repairing chemically damaged DNA
UvrA, B, C, and D recognize and remove a short segment of damaged DNA
DNA polymerase and ligase finish the repair job
base mismatch
another abnormality in DNA
structure of the DNA double helix obeys the AT/GC rule of base pairing
however, during DNA replication, an incorrect base may be added to the growing strand by mistake
DNA polymerases have a 3’ to 5’ proofreading ability that can detect base mismatches and fix them
if proofreading fails, the ? repair system comes to the rescue
MutS protein slides along DNA and finds a mismatch
MutS/MutL complex binds to MutH, which is already bound to a hemimethylated sequence
? repair systems are found in all species
important: these systems are specific to the newly made strand
molecular mechanism of ? repair in Ecoli
3 proteins: MutL, MutH, MutS
detect mismatch and direct its removal from the newly made strand
proteins are “Mut” bc their absence leads to a much higher mutation rate than normal
MutH can distinguish btw the parental vs daughter strand
prior to replication, both parental strands are methylated
immediately after replication, the parental strand is methylated, whereas the newly made daughter strand is not
mismatch repair
wrong base is paired during DNA replication
normally, DNA pol 3’ → 5’ exonuclease can proofread and fix mistakes, but if it misses one, ? comes to rescue
in E. coli
MutS scans the DNA and detects the msimatch
MutL joins MutS to form a complex
MutH already bound near the mismatch at a hemimethylated site (where only the parent strand is methylated)
MutH cuts the new (unmethylated) strand at the mistake site
MutU unwinds the DNA
an exonuclease removes a section of the strand, including the mismatch
DNA pol adds the correct nucleotides
DNA ligase seals the gap
note
MMR occurs after replication to fix remaining errors
only the newly made strand is corrected (recognized by lack of methylation)
proteins involved are termed Mut bc their absence leads to a much higher mutation rate than normal
DNA double strand breaks
VERY DANGEROUS
breakage of chromosome into pieces
caused by ionizing radiation and chemical mutagens
also caused by reactive oxygen species which are the byproducts of cellular metabolism
10-100 breaks occur each day in a typical human cell
breaks can cause chromosomal rearrangements and deficiencies
they may be repaired by
homologous recombination repair HRR
nonhomologous end joining NHEJ
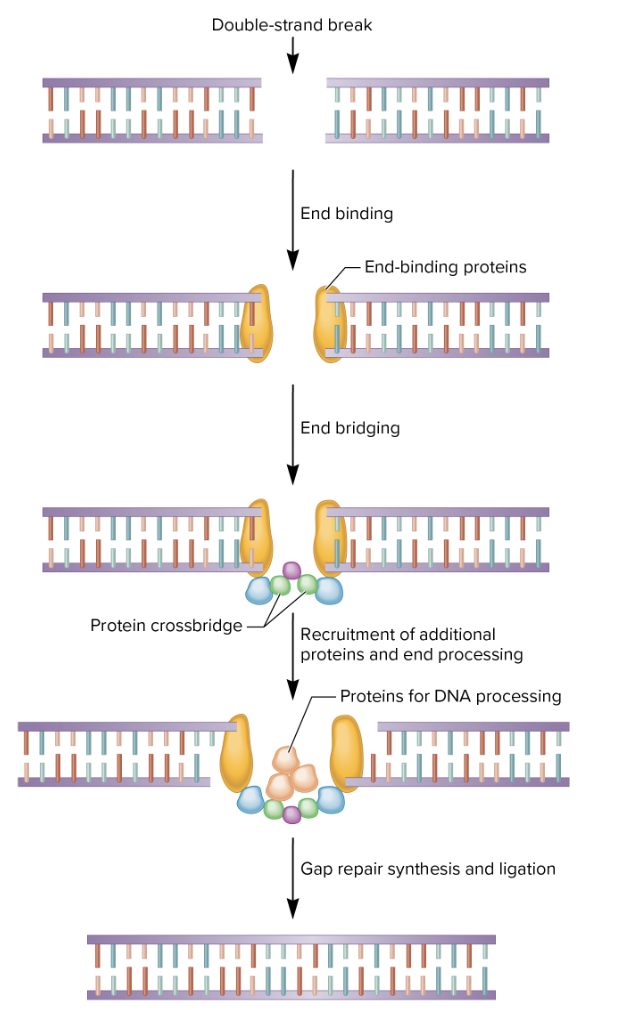
nonhomologous end joining
broken ends are recognized by end-binding proteins
formation of crossbridge
processing may result in deletion of a small region
not error free
fixes dangerous double stranded breaks in DNA (both strands are broken)
doesn’t need a template (unlike homologous recombination repair- other method to repair double stranded breaks)
steps
special proteins bind broken ends of DNA
proteins from crossbridge to keep ends close together
extra/damaged DNA is trimmed; more proteins recruited
DNA pol fills in missing bases, DNA ligase seals the break
fast, but error prone
used in non-dividing cells or when no template available