3. Atomic Theory, Millikan's Oil Drop & Radioactivity
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
who was involved in the battle of the greek philosophers
aristotle (350 BC)
democritus (400 BC), plato
(^older theories)
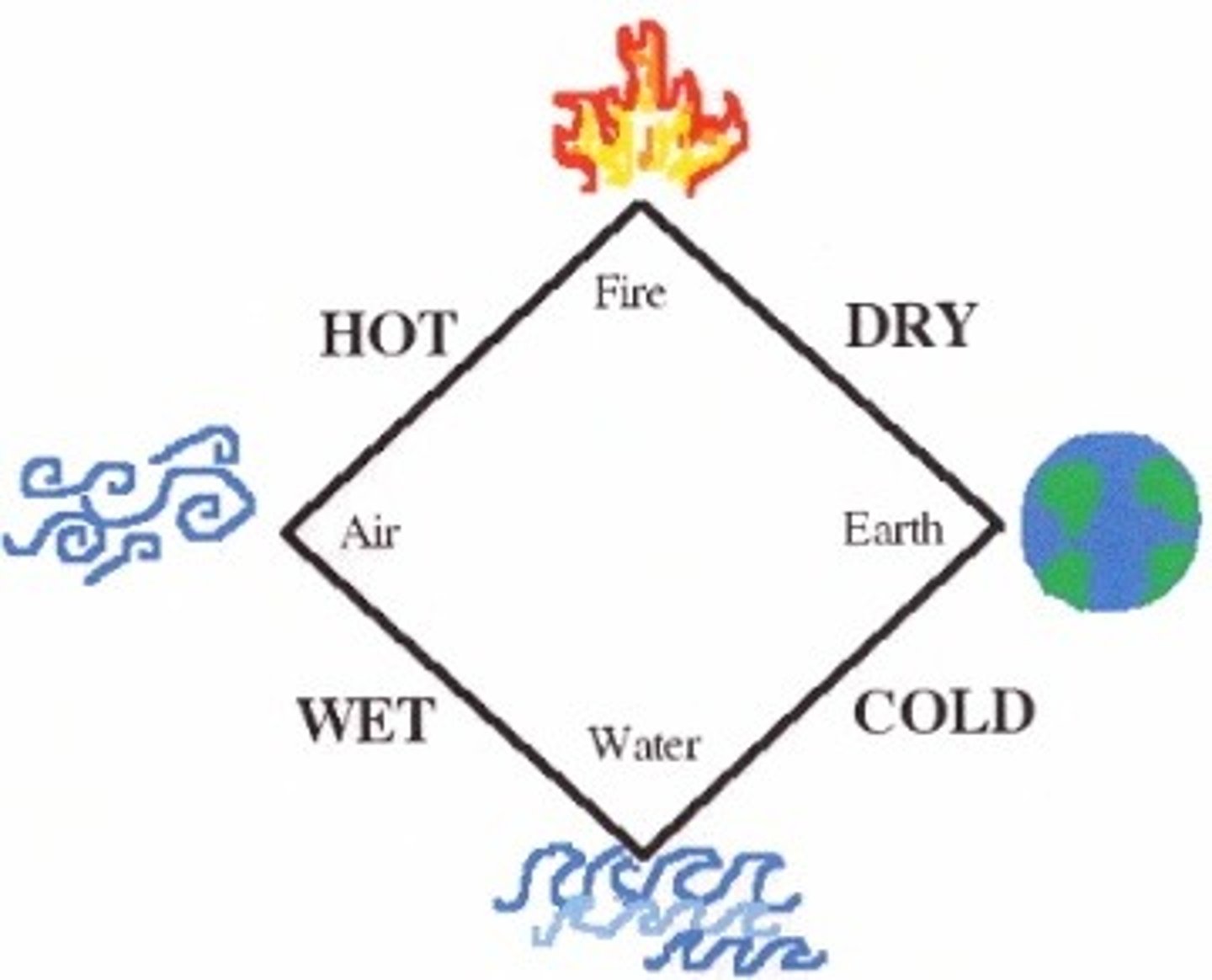
3 parts of Aristotle's beliefs
1) matter made of 4 classical elements of earth, water, fire, wind that determined people'es personality
2) elements had primary, secondary properties of hot and cold, wet and dry
3) matter was continuous (same all throughout inside) with no smaller partcles inside
Democritus (400 BC) - main idea
- if you continually cut substance in half, you reach an undividisble particle = matter made of tiny indivisible particles > aka atom
(atomus = indivisible)
- originated our modern idea of atom
Democritus' theory errors (3)
1) thought that all substances have their own tiny particles (tree atoms vs rock atoms)
2) didnt recognize similarities between particles of diff substances (thought all were distinct)
3) his theory of the indivisible particle couldnt be tested
who won the battle of the greek philosophers + why
aristotle won bcs he was more popular and democritus' more accurate ideas were forgotten for 2000 yrs
what could be some reasons why democritus' ideas were forgotten for so long
trying to figure out how things work was ocnsidered ot be going against religion > considered heretic
- ppl preoccupied with survival while wars happening + plagues > abstract sci not so important
first scientist to develop atomic theory and when
john dalton (1807)
which 3 laws did Dalton's theory explain + meaning of the laws
1) Law of Multiple Proportions - elements in bonds can combine in diff ratios (ie: CO2 vs CO)
2) Law of Definite Composition - all samples of a given chemical compound have the same elemental composition (water from anywhere will have the same elements in compostion)
3) Law of Conservation of Mass - reactants = products
John Dalton's atomic model
Billiard Ball Model
supported democritus:
- all matter made up of atoms (indivisible, tiny)
- all atoms of an element = identical
- atoms of different elements = different
- atoms can combine in simple whole number ratios
(tldr: atoms = solid, featureless, spherical)

which points of Dalton's theory were incorrect + why
1) that atoms were indivisible > wrong bcs atomic bomb proved it was possibel to split the atom + subatomic particles exist
2) that all atoms of an element are identical > wrong bcs isotopes exist where atoms of same element have diff # of neutrons so have diff mass #
how did the development of the atomic bomb bring positive applications for sci & isotopes
proving that atom could be split gave us concept of nuclear energy (energ yreleased from nucleus) > missisauga nuclear power plant
isotopes apps:
- used to target tumours (harnessing E of atom by understanding the nucleus >> nuclear medicine)
which scientist came after dalton + when
J.J Thomson (1897)
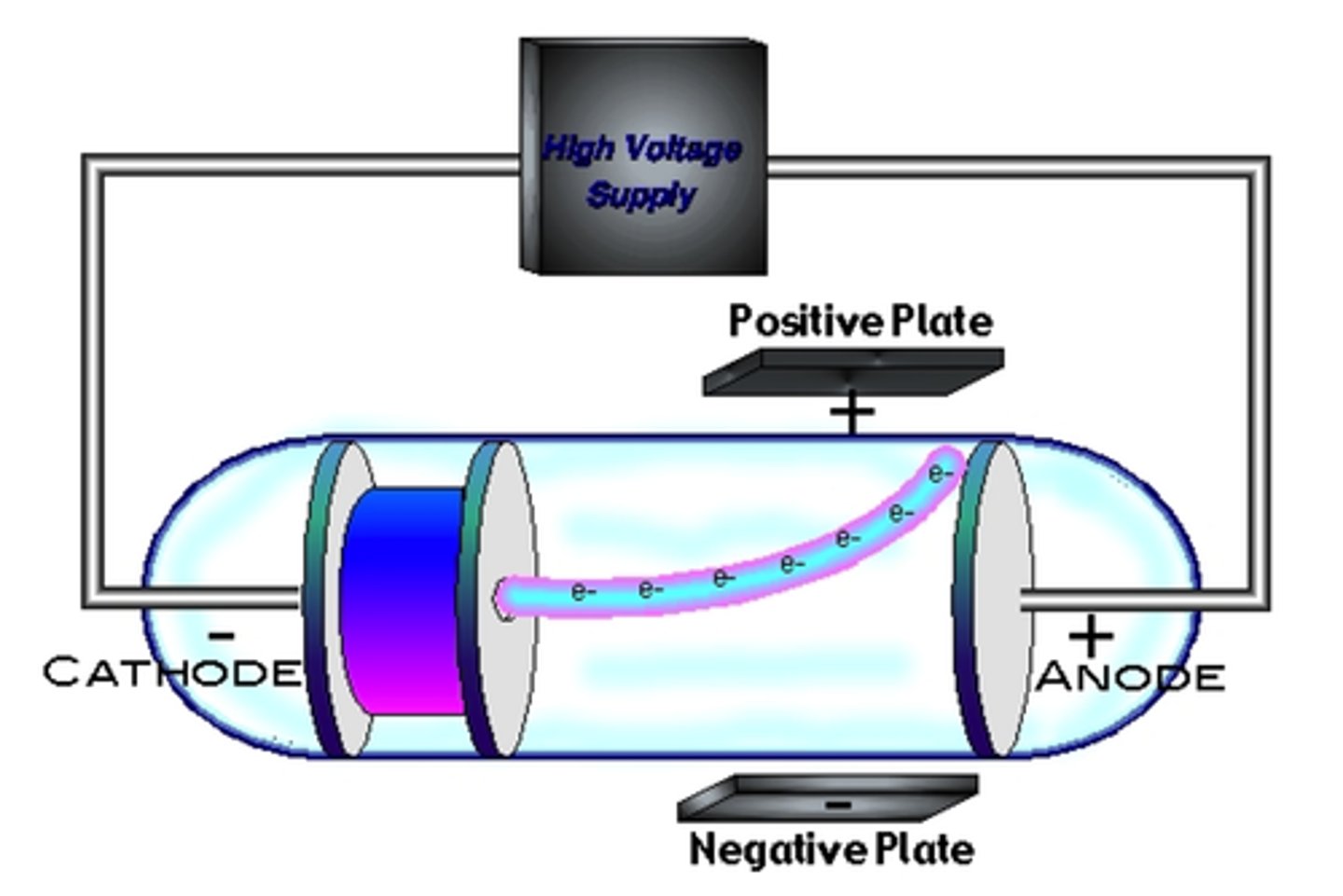
what experiment did JJ Thomson perform
cathode ray tube (CRT) experiment
- glass tube with vaccuumed gases at low pressure
- when electrciity applied to CRT > ray of light produced
process of cathode ray tube experiment + what was discovered
1) when (+) and (-) plates placed above and below CRT > cathode light ray deflected away from the (-) plate (repelled) and was atracted to (+) > so the particles of the ray were (-) charged
2) when magnetic plates placed above and below CRT, cathode ray deflected and moved according to the magnet > light can only deflect if magnetic feild acts on a particle with mass > so there was mass
= discovery of e-
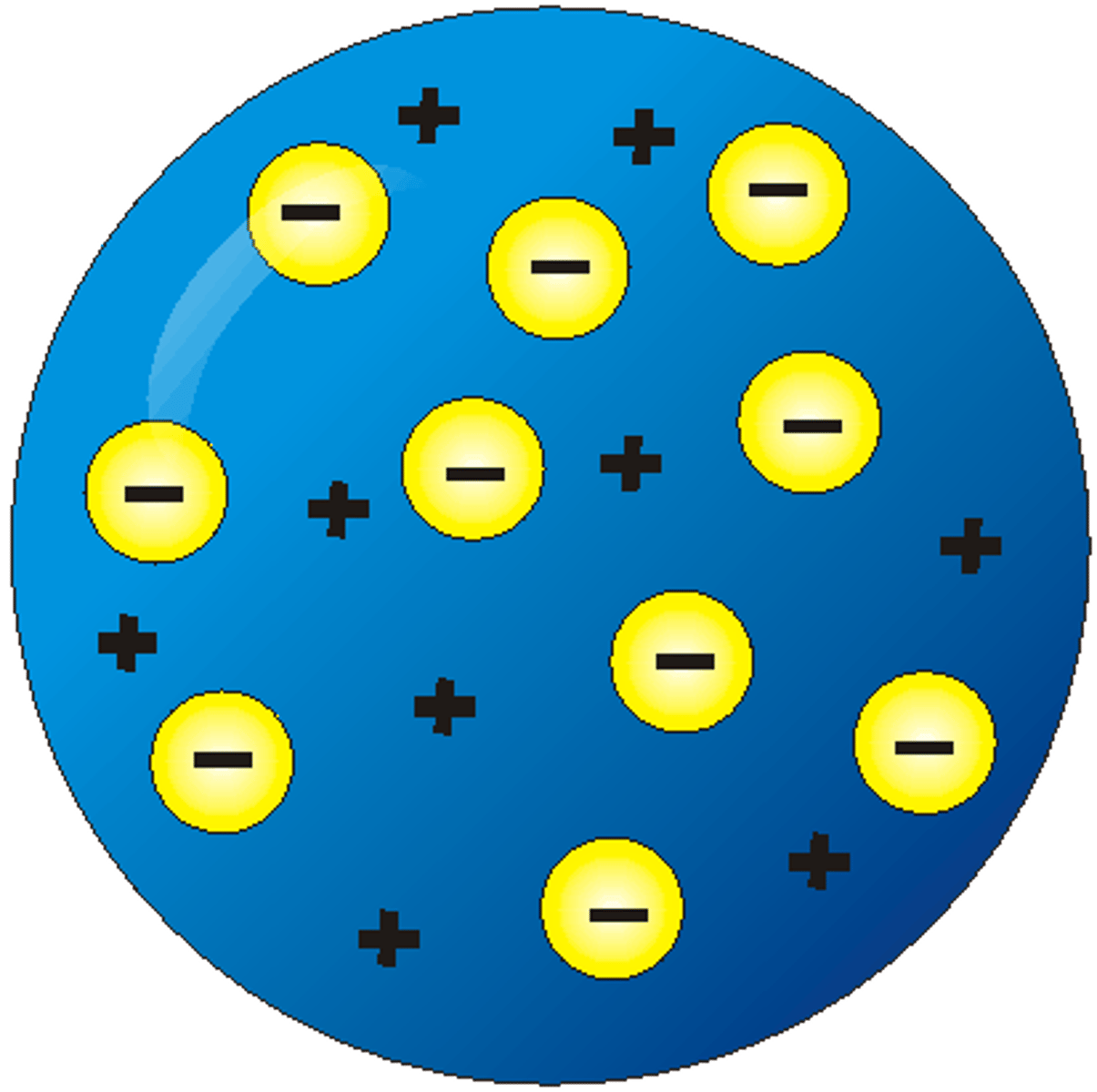
J.J. Thomson's atomic model
- plum pudding /raisin bun
theory:
- spherical atoms have negatively charged electrons
- atoms are neutral so rest of atom = positively charged empty space
- negatively charged electrons randomly dispersed throughout atom like raisins
sequentially who came after thomson and what did he do
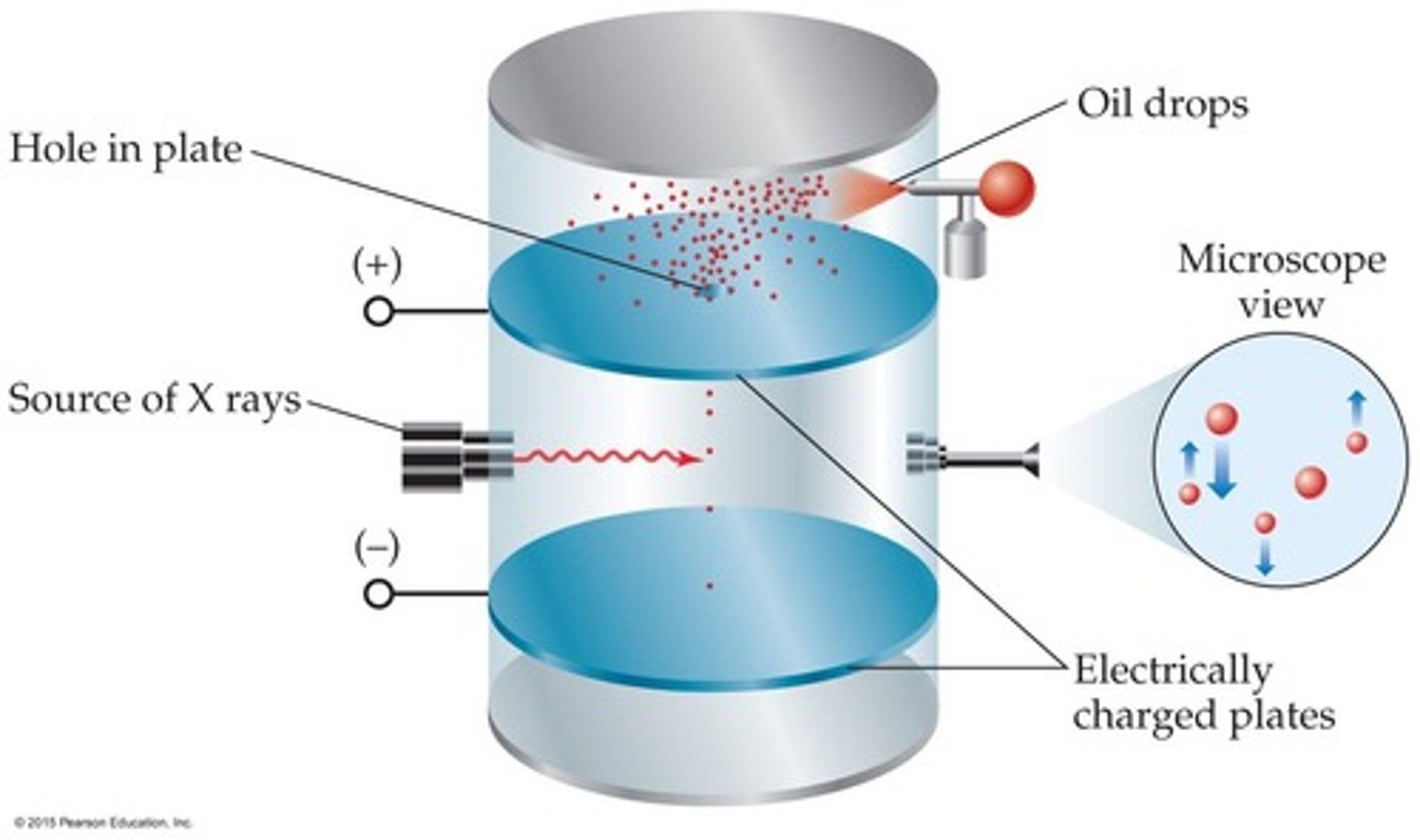
robert millikan and the oil drop experiment (1909)
- determined teh charge of an e- and the charge to mass ratio
explain milikan's oil drop experiment
- inside a tube chamber, there are 2 charged plates, a (+) plate at the top which has a hole in it, and a (-) plate below
- oil drops are sprayed into the chamber and fall thru the hole down onto the (-) plate
- x-ray source bombards x-rays into the chamber to give oil drops energy > produces (-) charge on the drops
- oil is dense so gravity pulls it down as it falls thru the hole > eventually drops slow down and start going up in the reverse direction
^^ bcs the drops are (-), they repel (-) plate below and attract up to the (+) plate but gravity still exists so they end up suspended in the middle
- through math, he figured out the exact mass annd charge the e-'s would need to float
which scientist came after thomson + era
ernest rutherford = 1911
why did rutherford use gold for his experiment
alpha particles = big > easier to block
but gold can be made thin enough for alpha particles to penetrate
and gold = very stable > wont corrode easily
Rutherford's Experiment (prediction)
gold foil experiemnt/alpha scattering experiment
- tested Thomson's theory to see if atom was acc mainly empty space by aiming positively charged alpha particles at a thin sheet of gold foil
- prediction: if atom = (+) empty space > tiny (+) charged atoms shot at gold foil would all pass right through
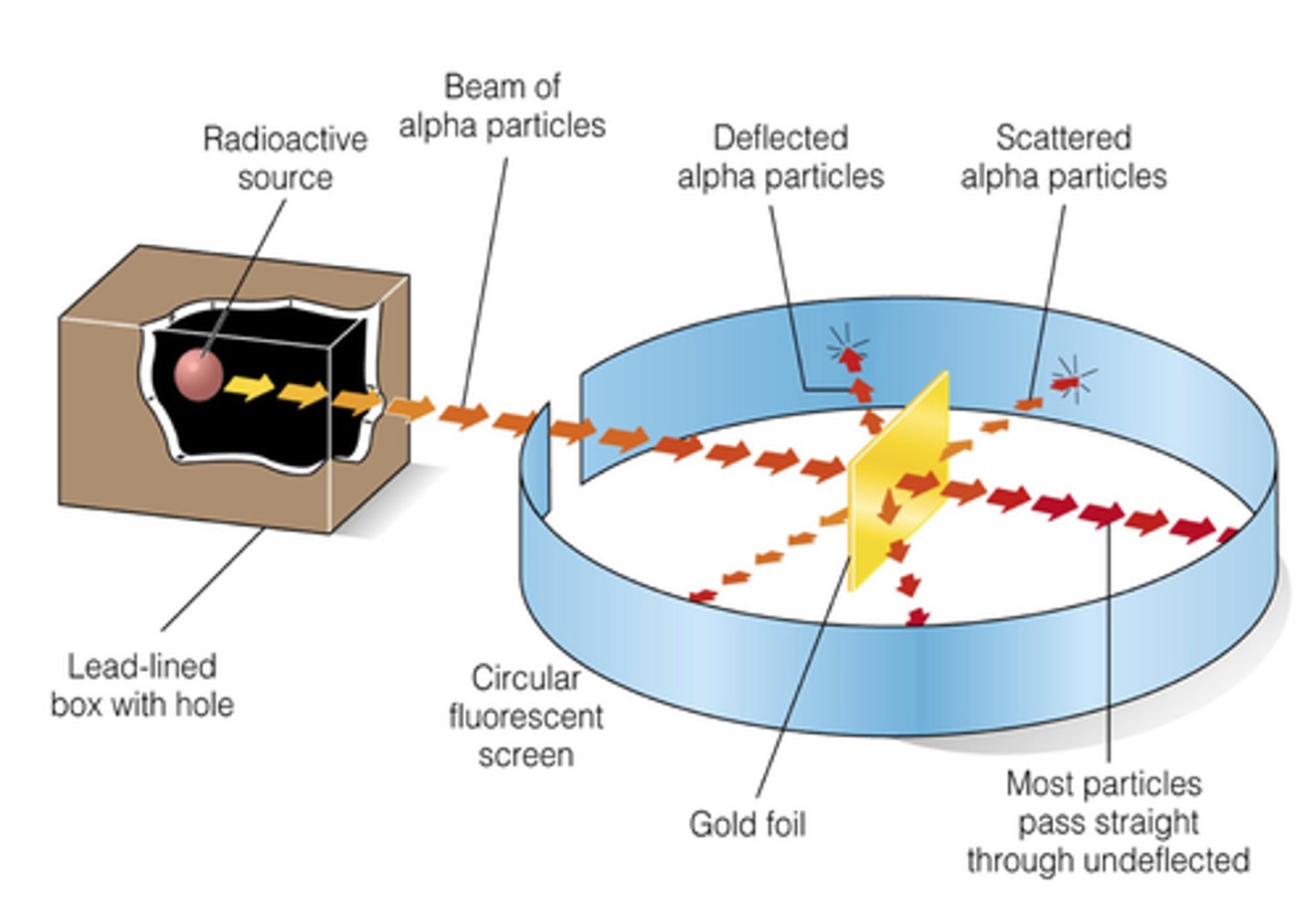
results of gold foil experiment + why
- most particles passed straight thru, some deflected at angles, and 1/2000 reflected back to origin
- most passed thru bcs MOST of atom = empty space
- the 1/2000th reflected backwards rays are bcs it hit smth hard inside foil = tiny dense core ~ nucleus
- the rays that deflected are bcs alpha particle = (+) so when it got close to (+) nucleus > repelled and deflected
APP: how did rutherford know the alpha particles didnt reflect backwards bcs they had collided into the randomly scattered e-?
bcs the mass of e-'s is insignificant so it would not have the ability to stop the high speed alpha particles
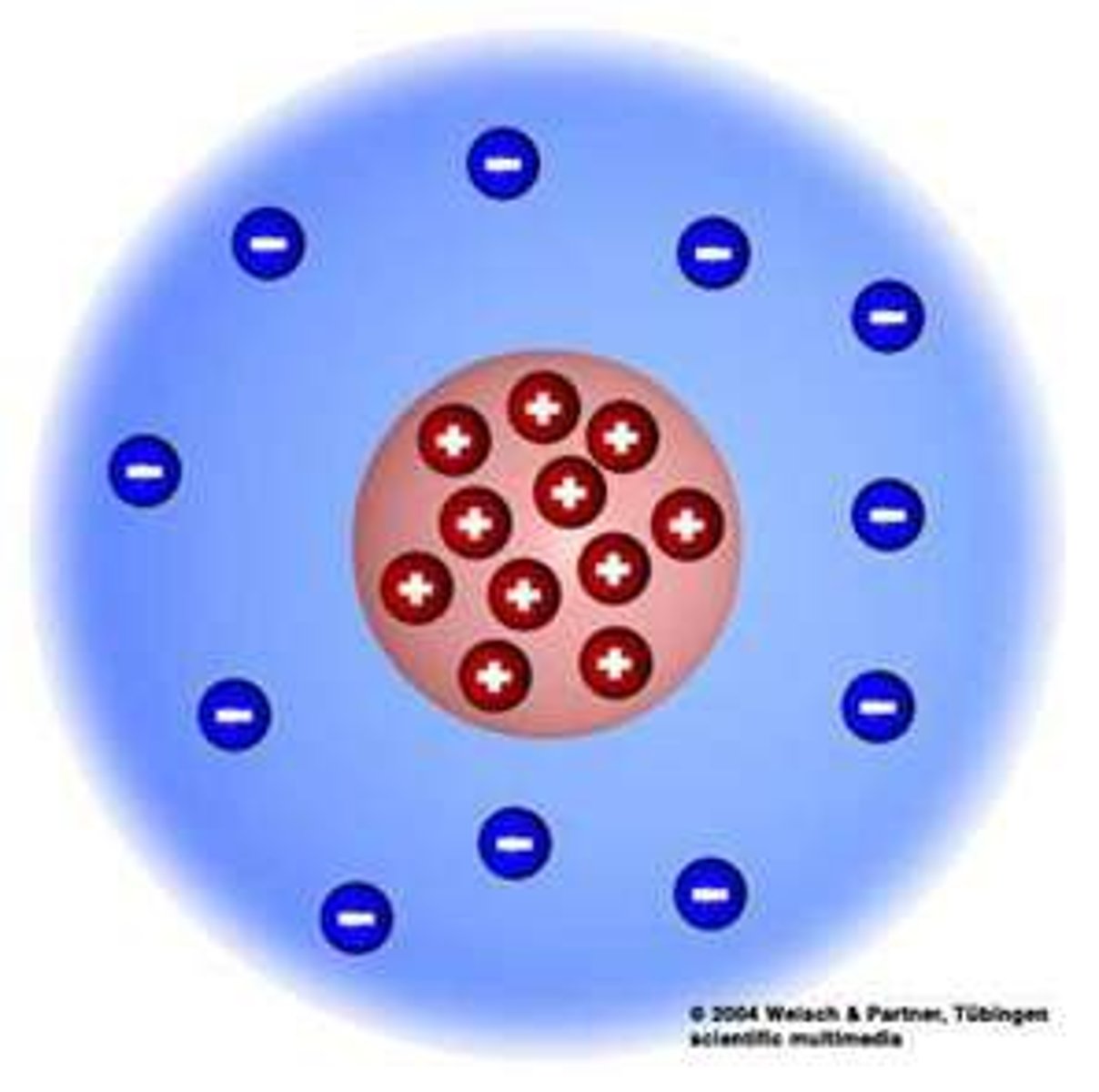
Ernest Rutherford's atomic model
nuclear model, solar system model, alpha scattering model
updated theory:
- atoms are tiny, round, mainly empty space
- has a dense centre called nucleus where mass and positive charge are concentrated
- electrons thought to orbit nucleus like planets orbit the sun
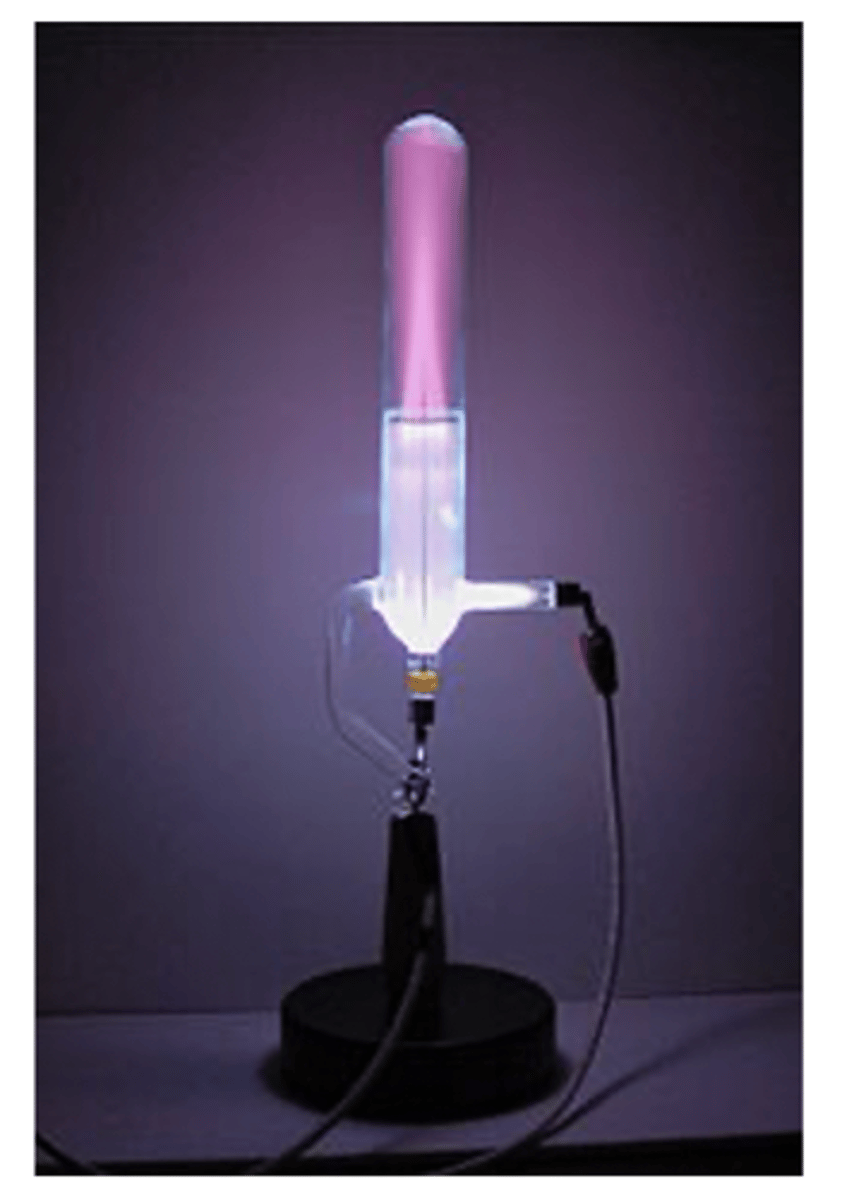
how was the proton discovered + by who
- thru modified CRT: canal ray tube
- they put Hydrogen inside tube and 1e- went down in tube (green glow) and the other 1p+ went up (purple/red glow)
- experiment done by Eugene Goldstein but confirmed by Rutherford in 1919 > realized this is why nucleus was (+) charge
which scientist came after rutherford + when
james chadwick (1932)
what did james chadwick discover + how
- realized mass discrepancy in helium: had a mass of 4 but only 2p+ (mass of protons + e- didnt add to mass of atom)
-logic: electrons = no mass, protons = 1/2 of atom's mass,
therefore another particle at play with similar mass to proton but no charge
- discovery of neutron
- nucleus contains (+) protons & neutral neutrons
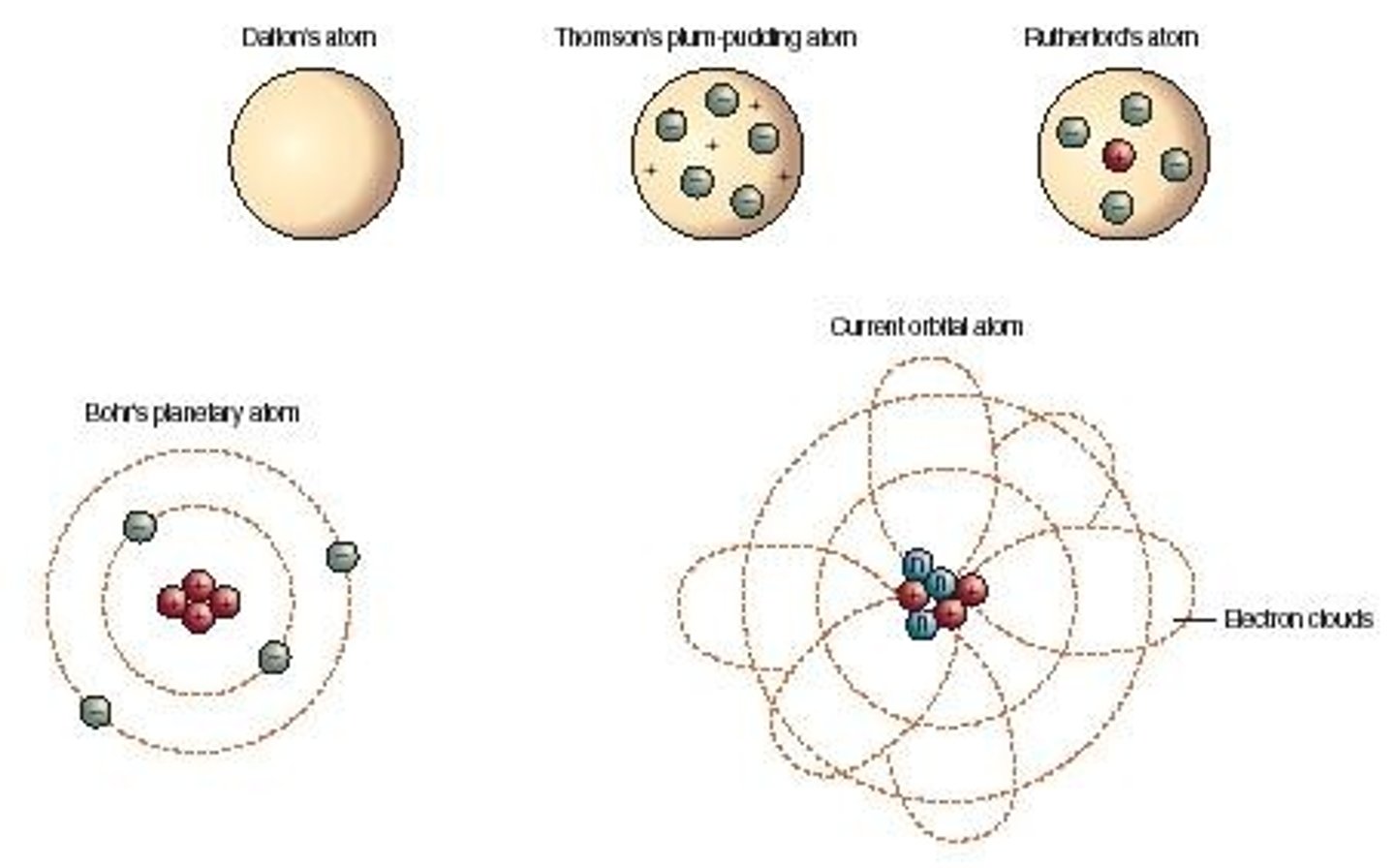
evolution of the atom
dalton - featureless sphere
thomson: e- randomly scattered in (+) space
rutherford: positve nucleus with e- scattered ~~ and then later planetary model with bohr: e- orbiting nucleus
why do we learn atomic theory if there are mistakes in it
- laid basis of current understanding of atomci theory
- shows scientific method at play with theories being developed and disproved
proton summary
(+) charged particle in nucleus
mass of 1 u
electronci charge = + 1.602x10^-19
electron summary
(-)
orbits nucleus
mass = 1/1837th u
electronci charge = - 1.602x10^-19
neutron summary
has no charge
found in the nucleus of an atom
mass = >1 u (bcs combined mass of 1p+ and 1e- makes it greater than 1)
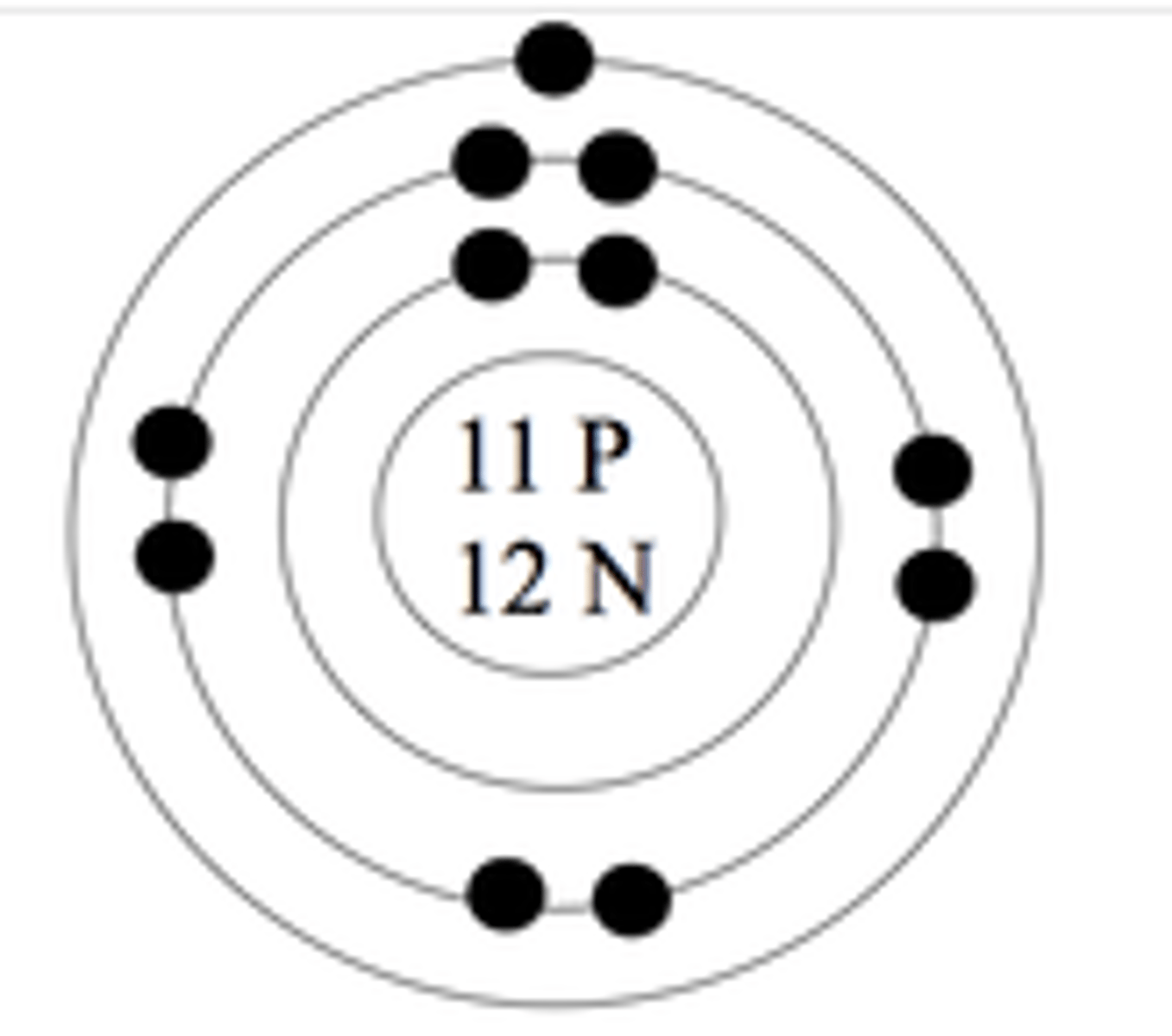
Bohr Diagram
combines bohr and rutherford models to show arrangement of e- around nucleus
- first shell = 2max
- every other shell after = 8max
standard atomic notation + how to find # of subatomic particles
note- atomic mass is always whole number
# of protons = atomic #
# of e- = # of p+ in neutral atom
# of n = mass - atomic #
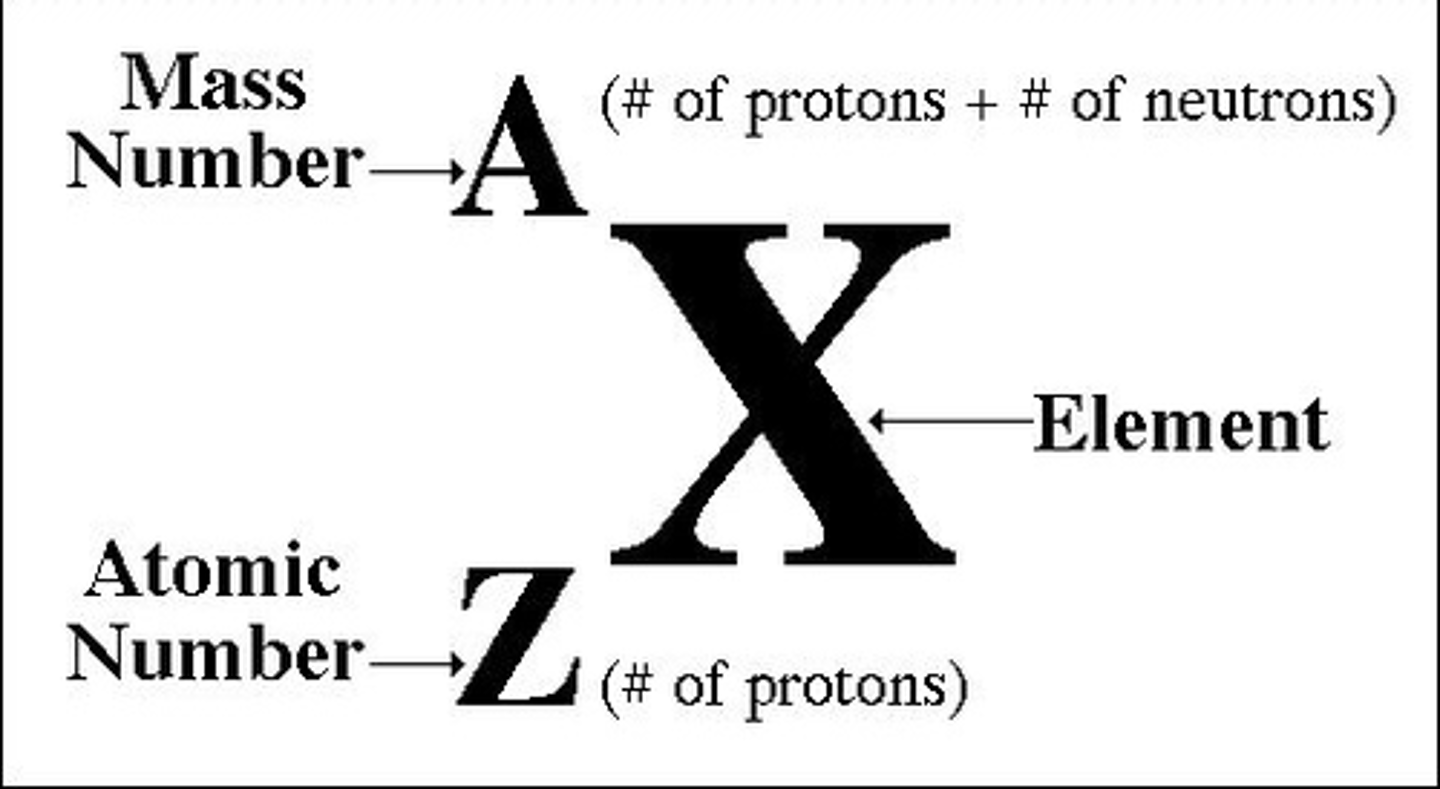
what unit is used to measure diameter of atom
angstroms
(symbol in pic)

who discovered radioactivity & when
Henri Becquerel in 1896
summarize the 3 types of radiation discoevered by rutherford (charge, size, danger)
1. alpha: (+) particles, least danger (stopped by clothing), 7400x more massive than Beta
2. beta: (-) particle (e-), middle danger
3. gamma: not a particle, very high E, most dangerous (destroys DNA) doesnt exist on earth