CHEM 203 - Intro and Gasses
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
qhag is the state equation as a function?
P = f(V, n, T)
Ideal gas law equation
PV = nRT
Moles are one way of expressing concentration. How many # molecules would be considered one mole?
6.02214076 × 10 ²³
Most physical quantities can be brooen down into what fundamental units?
MLT - mass, length, time
Example units: g, cm , s
Molecular weight =
m / n
(G/mol)
Molecular concentration symbol and equation
M = n/v
(mol/L)
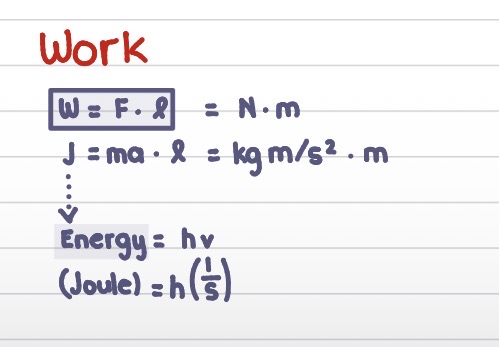
Work equation unit break down
Work is also equal to energy. What’s the energy equation?
W = F*m (N*m)
Also = ma * l (kg*m/s²*m)
= En = hv
(J = h(1/s))
What numbers (with units) did these standard units come from?
/
m = c speed of light
kg = h Phlanck’s Constant
mole = Avogadro’s number

What causes water to not boil at 100 C in YYC?
What is the equation for it? What’s the proportionality?
Pressure changes water’s boiling point here, it is specified when describing Celsius.
Pressure = Force / Area
Lower A = higher P
3 ways to change state of equilibrium (gas in rectangle with moveable wall photo example)
Work (on or by a system such as moving a wall)
Heat (energy transfer)
Increase or decrease gas concentration
First law of thermosynamics equation
Bottom line

Can the law of energy conservation be proven or disproven?
No
Gasses in rectangle with moveable wall example:
1) After an inward wall push towards the gas, what would be the next state of equilibrium in this isolated system? Describe it in an equation form
2) how about in a closed rectangle with a middle wall and gas particles on either side?
3) situation above but a rectangle divided into 3?
What type of equilibrium is this?
When P ext = P int
P left = P R
P A = P B = P C
Pressure - mechanical equilibrium
What lowers freezing point of water?
Salt
Thermal equilibrium: in a rectangular container with an immovable wall and the left side is hotter, what happens to energy to reach thermal equilibrium? Which law of thermodynamics is this?
En shifts to the right side until temperatures are the same
0th law developed after first and seconnd
molecular weight =
m / n