Lab Quiz 2 - Density, Recrystalization and TLC
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
26 Terms
density (p)
m/v
recrystalization is a chemical purification technique characterized by
the dissolving of an impure compount in a suitable solvent
recrystalization encourages the formation of
pure compound as crystals
recrystalization - dissolve ______ solid in ___ solvent, then let ____ to recrystalize
impure, hot, cool
most reactions go from starting material and reagant to
starting material, reagant, desired product, unwanted side product
acetanilide
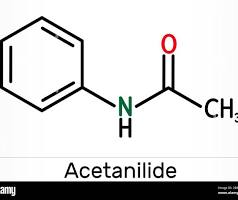
water solubility of acetanilide at 0 degrees celcius
0.5g/100ml
water solubility of acetanilide at 100 degrees celcius
5.5g/100ml
skills used
dissolving sample in water, filtration with buchner funnel, weighing
heating and stirring makes solution
uniform, homogenous
vacuum filtration is for
separating the filtrate
mixed solvent recrystallization example
trans - cinnamic acid
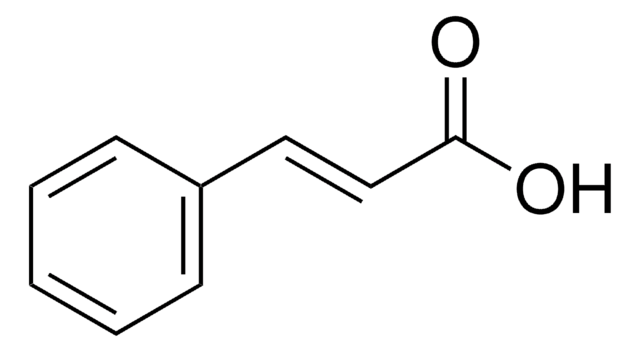
all compounds of impure trans-cinnamic acid dissolve in ________. add drops of water to form _________.
methanol, crystals
techniques to purify a compound if it doesn’t crystalize
Column purification, thin layer chromatography
Column purification utilizes the ______ phase vs __________ phase
mobile, stationary
example of mobile vs stationary phase
hexanes could be in a mobile phase while silica gel could be in the stationary phase
example for chromatography separation
my column purification!!
two compounds A and B. A in the mobile phase is eluted through B in the stationary phase. A moves faster than B and therefore they are separated
column chromatography steps
packing the column, loading the sample, eluting, collect
after purifying
run on a gel to see density
how do we quantify mobility?
retention factor (Rf)
Rf for TLC=
distance traveled by sample / distance traveled by solvent
nonpolar solvents
hexane, dcm, methanol
Thin layer chromatography - on a cillica gel the most polar sample travels the
Least
Thin layer chromatography - on a cillica gel the least polar sample travels the
Furthest
the desired compound will be the ____ polar
most
the impurities will be the _____ polar
least