Chemical bonding
1/8
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
What is covalent bonding?
the strong electrostatic attraction between a shared pair of electrons and the nuclei of the bonded atoms
What is ionic bonding?
the electrostatic force of attraction between oppositely charged ions formed by electron transfer.
What is a dative covalent bond?
A Dative covalent bond forms when the shared pair of electrons in the covalent bond come from only one of the bonding atoms
How is a sigma bond formed?
When two sp2 orbitals (one from each carbon) overlap to form a single C-C bond called a sigma σ bond
What is electronegativity
Electronegativity is the relative tendency of an atom in a covalent bond in a molecule to attract electrons in a covalent bond to itself.
What is metallic bonding?
Definition: A metallic bond is the electrostatic force of attraction between the positive metal ions and the delocalised electrons
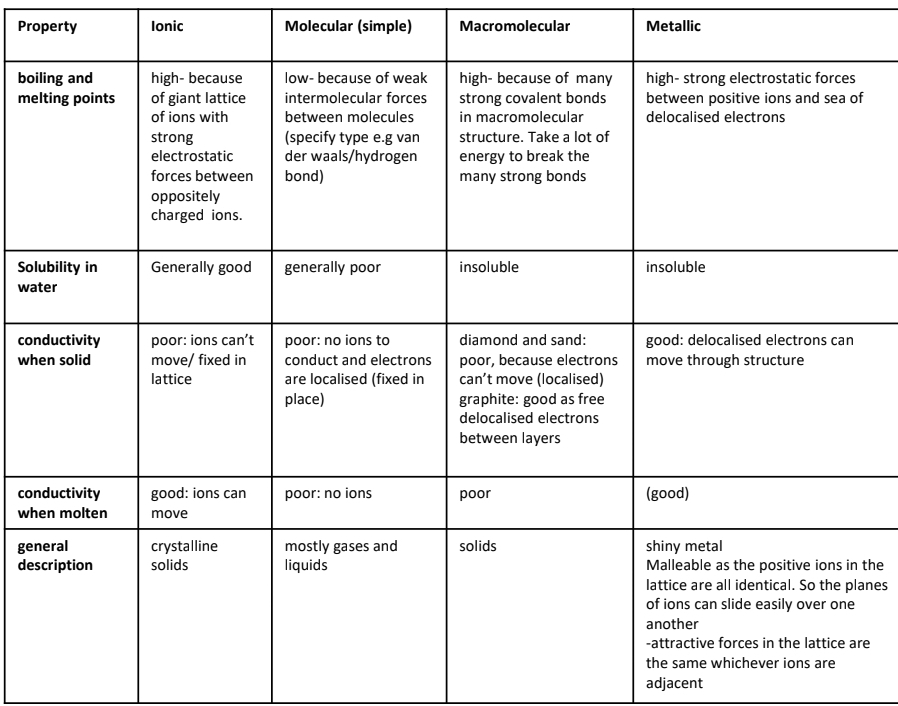
State the bond angle, No of lone pairs and No of bonding pairs in linear, trigonol planar and tetrahedral and trigononal planar, bent and octahedral and triginol by pyrimidal
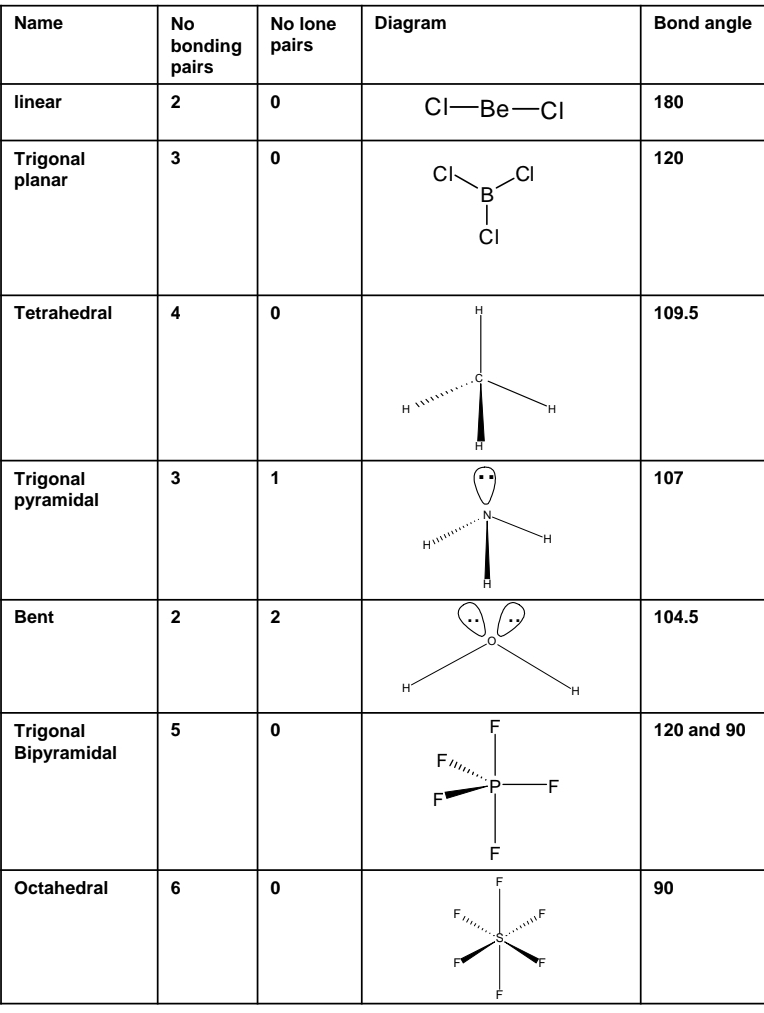
What is the bonding and structure in ionic,covalent and metallic bonding
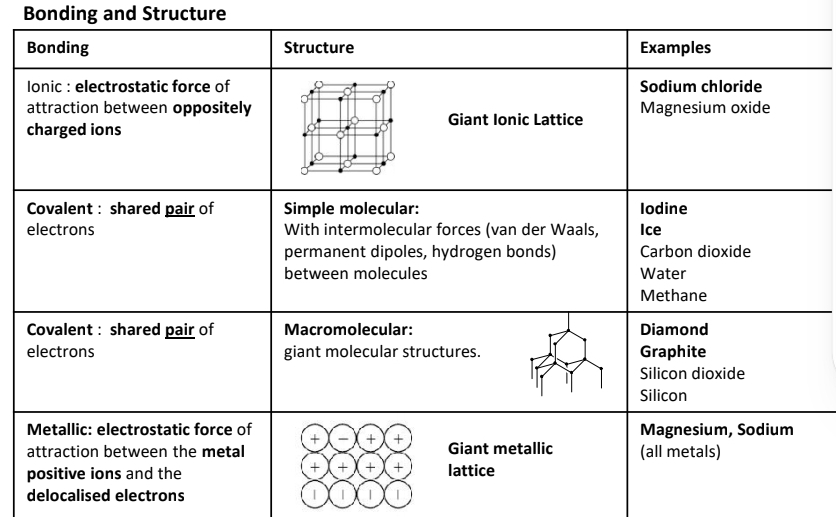
What is are the properties in ionic, covalent, molecular and giant molecular and metallic