CHM 243 - lewis structures & geometry
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
__________ on the period table must follow the octet rule. Common elements that must follow the octet rule include:
period 2
C, N, O, F
How do we determine what the "best" or "most acceptable" Lewis Structure is?
the best is the Lewis Structure with the smallest possible formal charges
on EACH ATOM
Draw bicarbonate’s ion structures
HCO₃⁻
Answer:
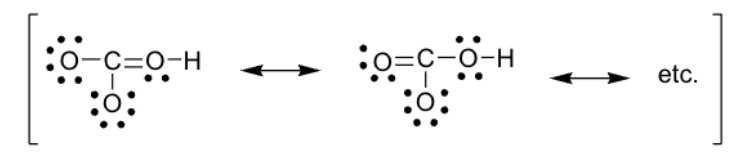
What is the formal charge of each atom of each of bicarbonate’s structures. Use this to find the best structure:
The structure to the right is the better structure, because 4 of those atoms have a formal charge of 0 (only the bottom O has a formal charge of -1)
The structure to the left only has 2 atoms with a formal charge of 0 (bottom O has FC = -1, left O has FC = -1, and right O has FC = +1)

In general, negative charges are BEST placed on atoms with __________ electronegativity, for example __________
Positive charges are BEST placed on atoms of __________ electronegativity, for example, __________
neg charges on high electronegative atoms
Examples: O, N, halogens
pos charges on low electronegative atoms
Example: metals
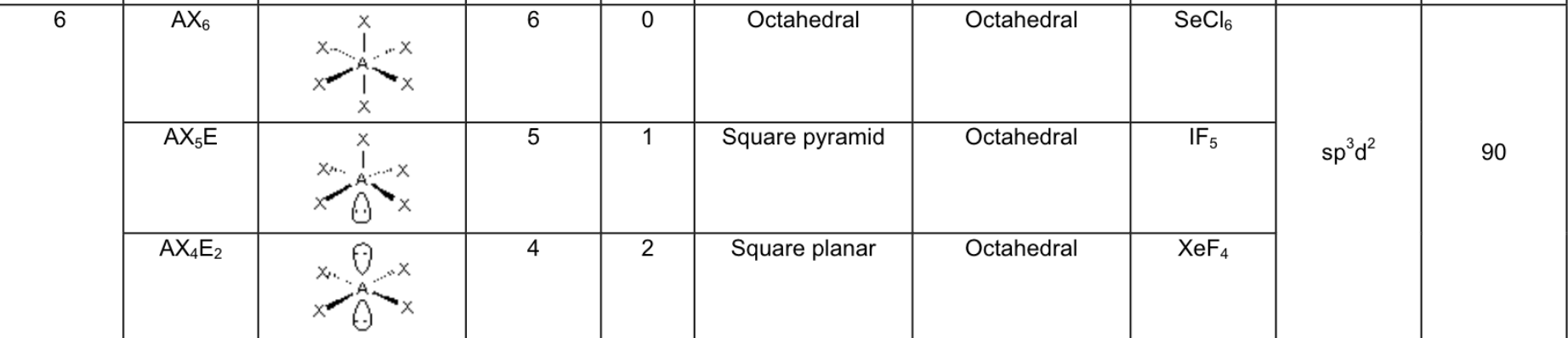
What are the 5 possible electron arrangements, and how many electron domains are there around the central atom for these arrangements?
Linear → 1 or 2 domains
Trigonal planar → 3 domains
Tetrahedral → 4 domains
Trigonal Bipyramid → 5 domains
Octahedral → 6 domains
If there are LONE PAIR electrons on the central atom, what does this mean for it’s geometry?
the electron geometry and molecular shape is the same
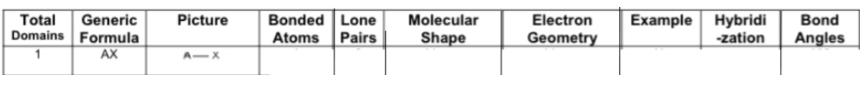
Fill in the chart:
Answer:
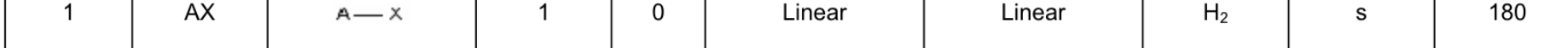
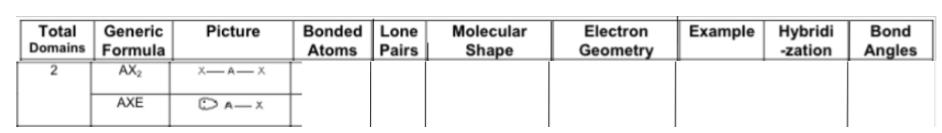
Fill in the chart:
Answer:

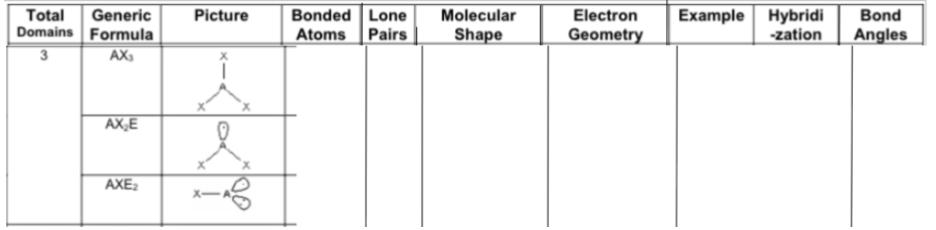
Fill in the chart:
Answer:
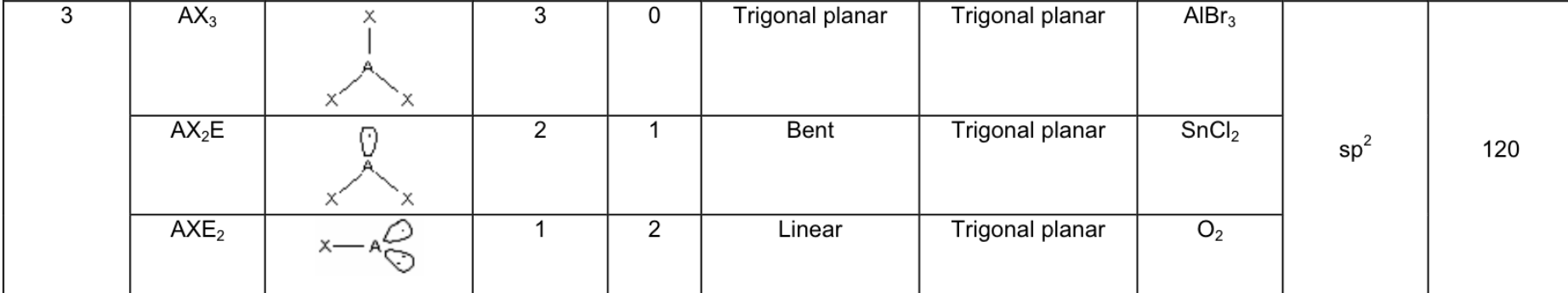
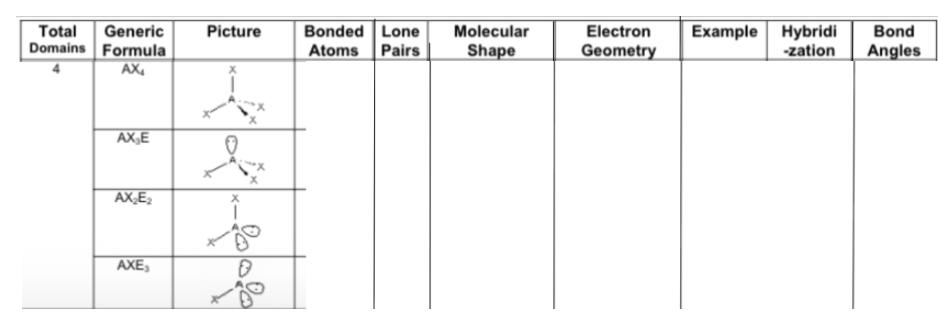
Fill in the chart:
Answer:
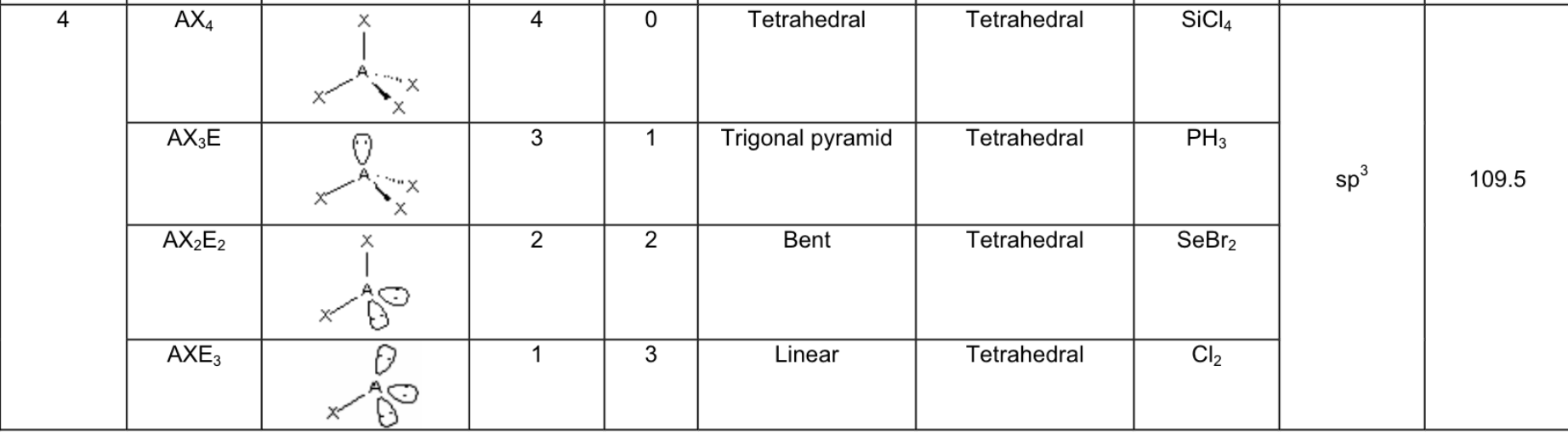
Fill in the chart:
Answer:
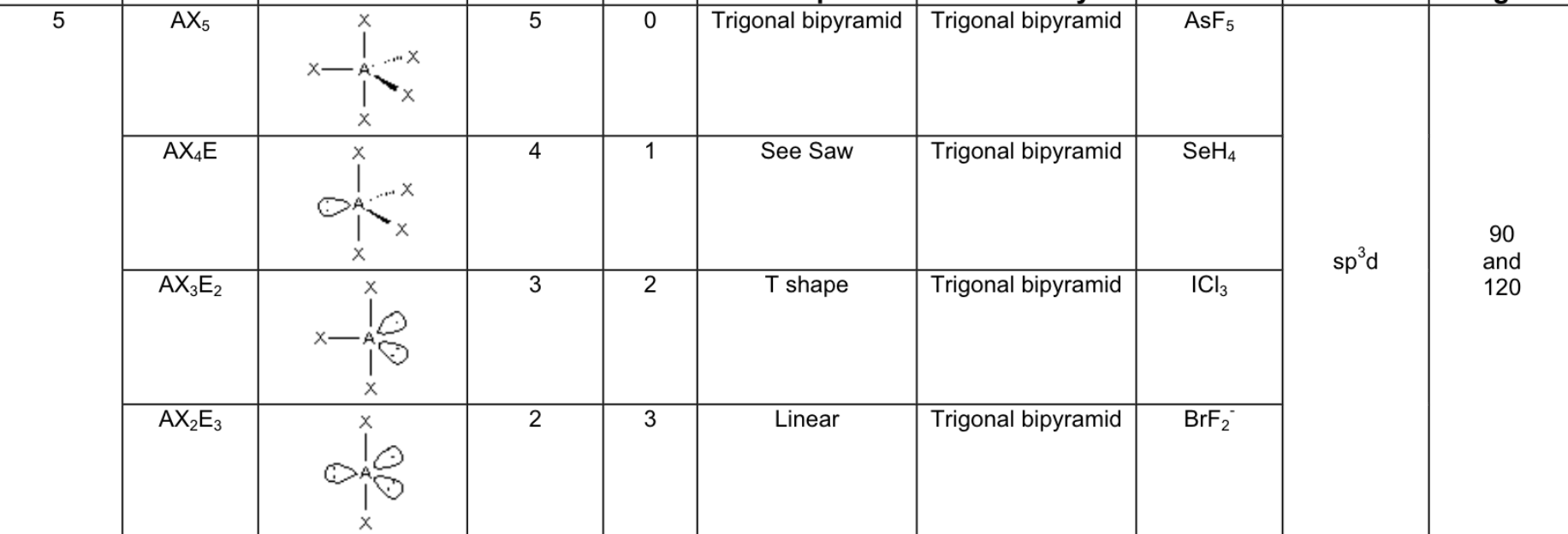
Fill in the chart:
Answer: