Chemistry U11: Unit 1
1/75
Earn XP
Description and Tags
Chemical Bonding, Matter, and Structures
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
76 Terms
Name the Following Compounds: Si2F6
Disilicon Hexafluoride
Name the Following Compound: Na3PO4 10H20
Sodium Phosphate decahydrate
Write the Chemical Formula for the Following:
MgCl2
NI3
NH3
H2SO4
Al(NO3)3
Hydrobromic acid
Potassium peroxide
Calcium hydroxide
Plumbic nitride
Magnesium chloride
Nitrogen triiodide
Ammonia
Sulfuric acid
Aluminum nitrate
HBr
K₂O₂
Ca(OH)₂
Pb3N4
Determine the number of protons, electrons, and neutrons in
a) 66Er 167
Protons = 68, Neutrons = 99, Electrons = 68
What is an isotope?
Atoms of the same element that have different mass numbers since they have a different number of neutrons in the nucleus.
What is a radioisotope?
Isotope with an unstable nuclei that undergoes radioactive decay.
Naturally occurring Boron consists if twi isotopes 19.60% 5B10 which has an atomic mass of 10.00u and 80.40% 5B11 which has an atomic mass of 11.00u. Calculate the average atomic mass of boron.
Final Answer: 10.80 u(rounded to 4 significant figures)
Iron has 4 naturally occurring isotopes of atomic mass (and abundance) of 53.940u (5.84%), 55.935u (91.68%), 56.935u (2.17%), and 57.933u (0.31%). Calculate the average atomic mass of iron.
Final Answer: 55.83 u(rounded to 4 significant figures).
How are the properties of metals and non-mentals different?
Metals are typically shiny, good conductors of heat and electricity, and are malleable and ductile. In contrast, non-metals are usually dull, poor conductors, brittle, and exist in various states at room temperature. Metals tend to lose electrons in reactions, while non-metals tend to gain or share them.
What are metalloids? Where are they found on the periodic table?
Metalloids are elements that have properties of both metals and non-metals. For example, they might be shiny like metals but brittle like non-metals, or conduct electricity only under certain conditions (making them useful as semiconductors).
They are found along the staircase-like line (zigzag) on the right side of the periodic table, between metals and non-metals. Common metalloids include boron (B), silicon (Si), arsenic (As), and tellurium (Te).
What is ionization energy?
The energy required to remove the first electron from a neutral atom in the gaseous state.
How does ionization energy relate to reactivity trends for metals? for non-metals?
L —> R across a period ionization energy increases.
T —> B down a group ionization energy decreases
Which would have larger ionization energies? Explain Why.
a) Na or Fr
b) Li or Ne
c) O or Te
a) Na, b) Ne, c) O
Which element has the larger atom?
a) Mg or Ba
b) Sc or Cu
c) Hg or Au
Ba, Cu, Au
Which of each pair below would have the larger atomic mass?
a) Mg or Ba
b) Sc or Cu
c) Hg or Au
Ba, Sc, Au
Which pair of atom(s)/ ion(s), has the larger radius?
a) Na or Ne
b) Ca 2+ or K+
c) Br- or Rb
Na, K+, Rb
How is a coordinate covalent bond different from a normal covalent bond.
In a normal covalent bond, each atom contributes one electron to form the shared pair.
In a coordinate covalent bond, one atom donates both electrons to the bond, often to an atom that needs more electrons to become stable (like a hydrogen ion, H⁺).
Despite the difference in how the bond forms, once formed, both types of bonds behave the same chemically.
Draw Lewis diagrams for the following molecules. State the shape and polarity of each.
a) CH2O
b) HCl
c) C2H2
d) H2S
e) CH4
f) NBr3
h) BeCl2
a) CH₂O (Formaldehyde)
Lewis Diagram:
mathematicaCopyEdit
O || H–C–H
Shape: Trigonal planar (around carbon)
Polarity: Polar (due to the C=O bond and asymmetry)
b) HCl (Hydrogen chloride)
Lewis Diagram:
CopyEditH–Cl ..
Shape: Linear
Polarity: Polar (large difference in electronegativity)
c) C₂H₂ (Ethyne/Acetylene)
Lewis Diagram:
mathematicaCopyEdit
H–C≡C–H
Shape: Linear (around each carbon)
Polarity: Non-polar (symmetrical molecule)
d) H₂S (Hydrogen sulfide)
Lewis Diagram:
CopyEditH–S–H .. ..
Shape: Bent
Polarity: Polar (due to lone pairs on sulfur)
e) CH₄ (Methane)
Lewis Diagram:
markdownCopyEdit
H | H – C – H | H
Shape: Tetrahedral
Polarity: Non-polar (symmetrical shape)
f) NBr₃ (Nitrogen tribromide)
Lewis Diagram:
markdownCopyEdit
.. Br – N – Br | Br
Shape: Trigonal pyramidal
Polarity: Polar (asymmetrical with lone pair on nitrogen)
h) BeCl₂ (Beryllium chloride)
Lewis Diagram:
CopyEditCl–Be–Cl
Shape: Linear
Polarity: Non-polar (symmetrical and electronegativity cancels)
Draw the Lewis Diagram for the ions or compounds: ClO4 -1, K2SO3, PCL4 +1, Cn-1, NO +1
Draw!!!
State the main type of intermolecular bonding which occurs between molecules of each of the following compounds:
a) PCl3
b) HBr
c) C2H4
d) BeBr2
e) SO2
f) HF
g) NH3
a) Type of bonding: Dipole-dipole. Why: Polar molecule with no hydrogen bonding.
b) Type of bonding: Dipole-dipole. Why: Polar molecule with a moderate difference in electronegativity (no H-bonding).
c) Type of bonding: London dispersion forces. Why: Non-polar molecule.
d) Type of bonding: London dispersion forces. Why: Linear and non-polar overall.
e) Type of bonding: Dipole-dipole. Why: Polar molecule due to bent shape.
f) Type of bonding: Hydrogen bonding. Why: Strong hydrogen bond between H and highly electronegative F.
g) Type of bonding: Hydrogen bonding. Why: Hydrogen is bonded to nitrogen, allowing hydrogen bonds.
How is a salt different from a covalent molecule?
A salt is an ionic compound formed when a metal transfers electrons to a non-metal, creating positive and negative ions that are held together by electrostatic attraction (e.g., NaCl).
A covalent molecule, on the other hand, is formed when non-metals share electrons to achieve stability, resulting in neutral molecules (e.g., H₂O or CO₂).
Is it possible for compounds to contain both ionic and covalent bonding at the same time?
Yes, these are called polyatomic ions
Compare the physical properties of ionic polar covalent and nonpolar covalent compounds.
Ionic compounds generally have high melting and boiling points, are solid crystals at room temperature, and conduct electricity when melted or dissolved in water. Polar covalent compounds have moderate melting and boiling points, may be liquids or solids, and can dissolve in water but usually do not conduct electricity well. Nonpolar covalent compounds usually have low melting and boiling points, are often gases or liquids at room temperature, and do not conduct electricity.
Types of bonding
a) Ionic
b) covalent
a) between metals and non-metals
b) between non-metals and/or polyatomic ions (VSPER) (Share electrons)
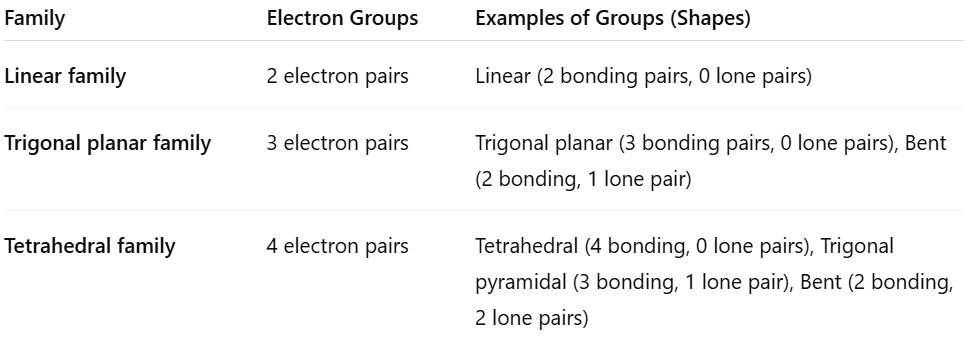
VSPER model
a) Two principles of the model
b) steps to predict the shape of a molecule
c) 3 VSPER families and groups within a family
a)Two Principles of the VSEPR Model
Electron pairs around a central atom repel each other and arrange themselves as far apart as possible to minimize repulsion.
Both bonding pairs and lone pairs of electrons affect the shape of the molecule, but lone pairs repel more strongly than bonding pairs.
b) Steps to Predict the Shape of a Molecule Using VSEPR
Draw the Lewis structure of the molecule.
Count the total number of electron pairs (bonding + lone pairs) around the central atom.
Determine the electron pair geometry by arranging electron pairs to minimize repulsion.
Predict the molecular shape by focusing on the positions of atoms (ignore lone pairs for shape name).
Consider the effect of lone pairs on bond angles and shape.
c)
Polar bonds and Electronegativity
a) determine the bond type (ionic, polar covalent, truly covalent) using electronegativity
b) determine the overall polarity (ionic, polar, nonpolar) of a molecule using molecular shapes
Here’s a clear guide for polar bonds and electronegativity:
a) Determining Bond Type Using Electronegativity Difference (ΔEN)
ΔEN Range | Bond Type | Explanation |
|---|---|---|
> 1.7 | Ionic | Electrons transferred, metal + non-metal |
0.4 to 1.7 | Polar Covalent | Unequal sharing of electrons |
< 0.4 | Nonpolar Covalent | Equal or nearly equal sharing of electrons |
b) Determining Overall Polarity of a Molecule Using Molecular Shape
Look at the bond polarities (from ΔEN) — are the bonds polar or nonpolar?
Consider the shape (VSEPR model) — do the bond dipoles cancel out?
If bond dipoles do not cancel, molecule is polar (has a net dipole moment).
If bond dipoles cancel out symmetrically, molecule is nonpolar.
If the compound is ionic, it’s considered ionic overall.
What is the Atomic Radius?
The measurement of the size of the atom. It is the balance between attractive forces that the nucleus has for the electron and the repulsive forces between the inner shell electrons. Atomic radius increases when we go down the period since we are adding shells. Atomic radius decreases from left to right.
What is Ionic Radius?
Electrons repel each other so adding an electron makes an atom larger, taking one away makes it smaller.
What is Electron affinity?
Opposite of electron negativity. Tells us how much an atom wants to gain an electron. Increasing direction from left to right and down to up.
What is Zeff?
Zeff is effective nuclear charge (hold electrons)
What is a Coordinate Covalent Bond?
When one atom donates BOTH electrons to a shared pair.
What is resonance?
Brining an electron with a negative charge to an electron with a positive charge. Molecules or ions that connot be accurately represented by just one Lewis structure.
How are Oxyacid’s attached in a Lewis Structure.
Hydrogen is always attached to the oxygen atom which is then attached to the central atom.
What is the Mass Spectrometer?
A tool scientists use to graph and identify unknown substances.
What is Average Atomic Mass?
The weighted average means that some atomic masses contribute more to the average atomic mass than the less abundant isotope contribute. The abundance of an isotope os reported as a percent/ Percent is determined by the spectrometer.
Average Atomic Mass Formula
AAM = (mass u)(% abundance) + (mass u)(% abundance)
Formula to find % Abundance
let x = fraction of Ga(2)
Then 1 - x = Ga(3)
(average amu)(x) + (amu average)(x-1) = Average Atomic Mass
What is Electronegativity
A number that decreases one atom’s ability to attract a bonding atom’s shared electrons.
Increasing (left to right) (bottom to top)
What is Dipole-dipole attraction?
Dipole-dipole attraction is the intermolecular force between the positive end of one polar molecule and the negative end of another.
Because polar molecules have permanent dipoles (regions of partial positive and negative charge), they attract each other like tiny magnets, causing molecules to stick together more strongly than nonpolar molecules do.
This force affects properties like boiling and melting points—molecules with stronger dipole-dipole attractions generally have higher boiling points.
What is Hydrogen Bonding?
Hydrogen bonding is a strong type of attraction between molecules that happens when a hydrogen atom is bonded to a very electronegative atom like fluorine (F), oxygen (O), or nitrogen (N).
This makes the hydrogen slightly positive, so it gets attracted to a nearby electronegative atom in another molecule, kind of like a tiny magnetic pull.
It’s why water sticks together and has a high boiling point compared to other similar-sized molecules.
What is London Forces (LDF)
London forces (or London dispersion forces) are the weakest type of intermolecular attraction. They happen when the electrons in atoms or molecules temporarily shift to one side, creating a tiny, short-lived dipole.
This temporary dipole can induce a dipole in a nearby molecule, causing them to attract each other briefly.
London forces are present in all molecules but are the only forces acting between nonpolar molecules like nitrogen or methane.
What is Ion-ion attraction?
Ion-ion attraction is the strong electrostatic force of attraction between positively charged ions (cations) and negatively charged ions (anions) in an ionic compound.
This force holds the ions tightly together in a crystal lattice, giving ionic compounds their high melting points and hardness.
What are the Interparticle forces ranked weakest to strongest.
LDF, Dipole-dipole, H-Bonding, Ion-ion
Name or write the formular for each of the following compounds. (Test 1A)
a) Stannous nitride
b) Bromous acid
c) Hydroiodic acid
d) Sodium biphosphate
e) Potassium peroxide
f) NaOH
g) Mg(HSO3)2
h) Ca(NO2)2
i) k2SO4 2H2O
j) HClO
a) Sn₃N₂
b) HBrO₂
c) HI
d) NaH₂PO₄
e) K₂O₂
f) Sodium hydroxide
g) Magnesium bisulfite
h) Calcium nitrite
i) Potassium sulfate dihydrate
j) Hypochlorous acid
For the Following Elements: P, Ba, Ag, Cl. Arrange the elements according to the ranking:
a) Decreasing first ionization energy
b) Decreasing atomic radius
c) Decreasing electronegativity
a) Cl > P > Ag > Ba
b) Ba> Ag > P > Cl
c) Cl> P > Ag > Ba
Given the standard notation 57/26 Fe 2+, which of the following statement regarding Iron is true?
a) Iron atom has 26 protons/ electrons and 57 neutrons
b) Iron has an atomic mass number of 57 and an atomic number of 26
c) Iron has 26 protons and 28 electrons
d) Iron has 57 neutrons and 26 protons
Answer: B
In the periodic table, all the elements within the same group are arranged from top to bottom in the order of increasing (choose all that may apply)
a) Atomic number
b) Ionization Energy
c) Oxidation number
d) electronegativity
e) Atomic Radius
Answer: A and E
Which list is in the correct order, from the most reactive to least reactive? Cl, P, S.
a) P > S > Cl
b) Cl > S > P
c) Cl > P > s
Answer: B
Draw the Lewis Structures of FeCl2, BeF2, and Nitrite ion
a) no c.c. no resonance, b) no c.c, no resonance, c) c.c, 2 resonance
If an metal (element X) reacts with chlorine to form a compound with the formula XCl, then what is the expected formula for the oxide of element X (X reacts with oxygen)? Explain.
If element X forms XCl, this means X has a +1 charge, since Cl⁻ has a -1 charge and the compound is neutral. Oxygen typically forms a -2 charge (O²⁻), so to balance +1 from X and -2 from O:
You need 2 atoms of X for every 1 atom of oxygen, giving the formula: X₂O
A sample of Zinc has three isotopes (Zn64 63.93u 49.2%, Zn66, 65.93u, 28.7%, Zn68 67.92u %?). Determine the average atomic mass of this sample.
AAM = 65.4U
Arrange O2-, Mg2+, Na+ in order for increasing radius (smallest to largest).
O2+ < Mg2+ < Na
When comparing the Sodium atom (Na) with its ion (Na+) which one is bigger? Explain.
Na is larger.
The following elements are from the same period. Predict which of the following will have the greatest ionization energy. Element X: same family as helium, Element Y: has an oxidation number of 2-, Element Z: a member of the halogen family.
Element X will have the greatest ionization energy because it is a noble gas, with a full outer shell and tightly held electrons, requiring the most energy to remove one.
What type of bond will most likely form between elements A and B? Element A has an electron negativity of 2.16, and element B has an electronegativity of 2.94.
Polar covalent bond
Which of the following substances will be more likely to be insoluble in water.
a) CH4 b) NH3 c) Br2 d) NaCl e) NaCO3
Answer: A and B
Draw Lewis structure and full solution for H2SO3, BCl3, and Fe(NO2)2 ion.
Draw: Bookkeeping, C.C. Resonance
Hypochlorite and Hypochlorous
ClO-
HClO
Nitrite and Nitrous acid
NO2-
HNO2
Chlorite and Chlorous acid
ClO2-
HClO2
Sulfite, Bisulfite, Sulfurous
SO3 -2
HSO3
H2SO3
Phosphite, Biphosphite, Dihydrogen Phosphite, Phosphorous acid
PO3 -3
HPO3 -2
H2PO3-
H3PO3
Acetate and Acetic Acid
CH3COO-
HCH3OO
Nitrate and Nitric acid
NO3-
HNO3
Chlorate and Chloric acid
ClO3-
HClO3
Carbonate, Bicarbonate, Carbonic acid
CO3 -2
HCO3 -
H2CO3
Sulfate, Bisulfate, Sulfuric acid
SO4 -2
HSO4 -
H2SO4
Phosphate, biphosphate, dihydrogen phosphate, phosphoric acid
PO4 -3
HPO4 -2
H2PO4 -
H3PO4
Perchlorate and Perchloric acid
ClO4 -
HClO4