Infectious Diseases: Viral Diseases
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
Virusessease
a small infectious agent that can multiply only in living cells of animals, plants or bacteria; cannot reproduce or conduct metabolic processes without a host cell
• Parasites
• Vector-borne viruses multiply in both the invertebrate vector and the vertebrate host
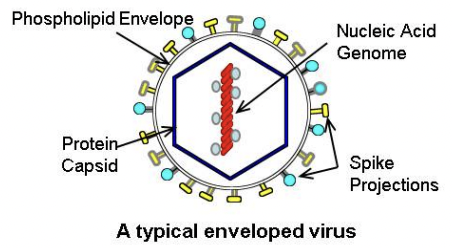
Virion
a complete virus particle that is the extracellular infective form of the virus; it includes the capsid (outer protein shell), the RNA or DNA core, and sometimes external envelopes
Virus classification
Viral genome can consist of:
• DNA
• RNA • RNA viruses include retroviruses such as HIV • RNA viruses, especially retroviruses, are prone to mutate
• can be single- or double-stranded
• can be linear or circular
• can vary in length
Baltimore Classification System
classifies viruses based on replication method and type of nucleic acid genome
retrovirus
Retroviruses use RNA for their genetic material, and after infecting a cell, the retrovirus converts its RNA to DNA, then integrates the viral DNA into the host cell DNA so it is replicated.
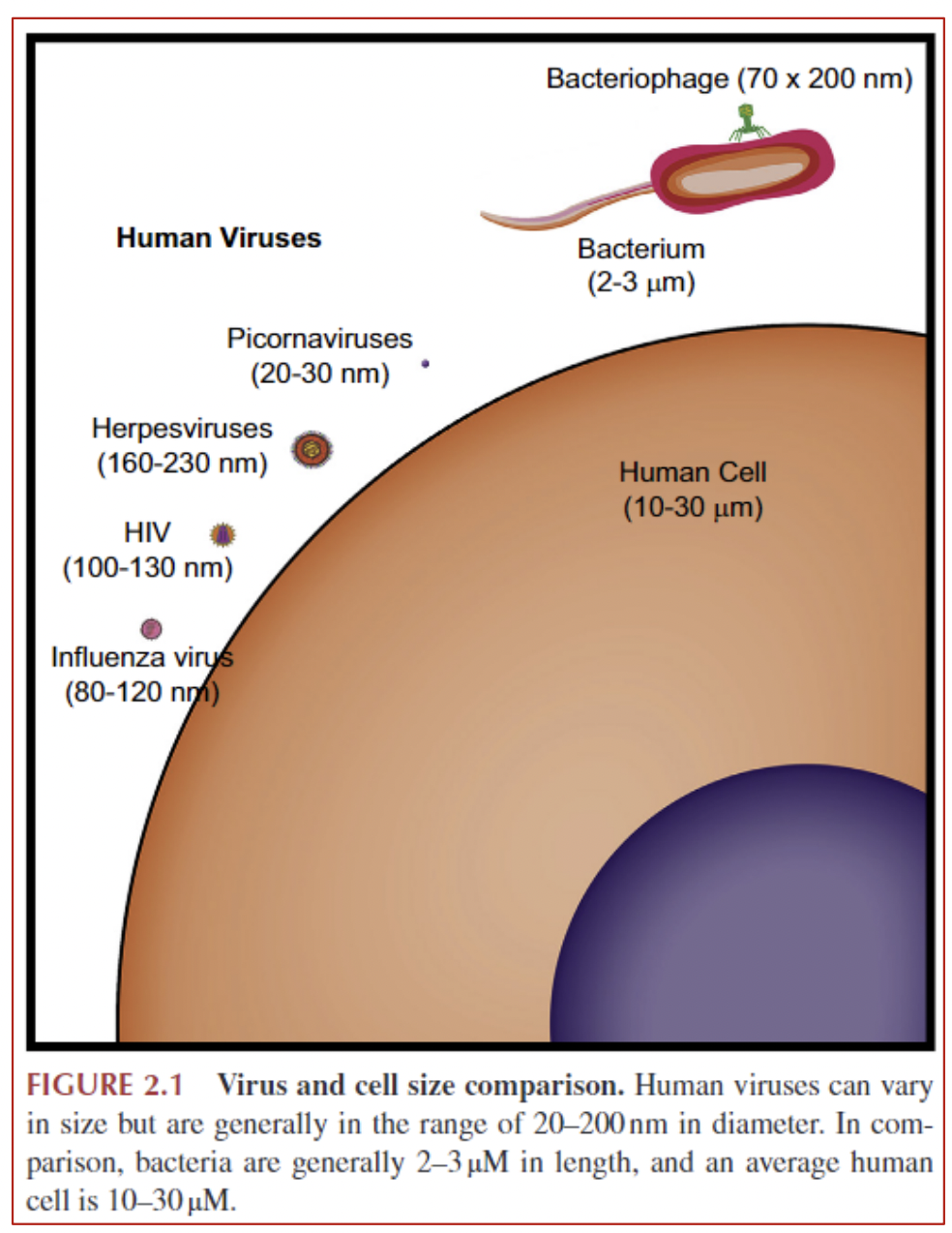
Viral interaction with host
• Viruses typically affect one type of cell • e.g., common cold viruses affect cells of upper respiratory tract
• penetrate the host cell membrane or cell wall & inject its genome into host cell
• forces the host cell machinery to replicate the viral genome
• The new viruses emerge from host cell (this kills the host cell)
• Latent infections
• Chronic viral infections
• Cancer
Latent infections
viral DNA or RNA remains in host cells but doesn’t replicate or cause disease for an extended time
• Latent viral infections can be transmissible during the asymptomatic period, so that person-to-person spread still occurs
• Ex.: herpes viruses
Chronic viral infections
Continuous viral shedding, e.g., with persistent hepatitis B or C
Cancer
some viruses don’t kill the cells they infect, but instead change cell function
• If this includes changing cell division, this can cause cancer
Common viral infections include infections of
• Respiratory tract • E.g., common cold, flu, pneumonia
• Gastrointestinal tract • E.g., norovirus, etc.
• Liver • E.g., hepatitis A, B, C
• Nervous system • E.g., rabies, West Nile, polio
• Skin • E.g., warts, chickenpox
• Placenta & fetus • E.g., Zika virus, rubella, cytomegalovirus
• Multiple body systems • E.g., enteroviruses
how viruses are transmitted
inhaled
swallowed
insect bites (vector borne)
sexual activity
exposure to blood
Viral Disease Management
• Viral diseases – not treatable with antibiotics
• Vaccines for viral infections
Vaccines for viral infections include
• Hepatitis A •
Hepatitis B
• Human papillomavirus
• Influenza
• Japanese encephalitis
• Measles, mumps, rubella
• Polio
• Rabies
• Shingles
• Yellow fever
• COVID-19
Use of fetal cells to produce vaccines against viruses
• A few vaccines are prepared by growing the viruses in fetal embryo fibroblast cells: • varicella (chickenpox) • rubella • hepatitis A • one version each of shingles and rabies
• These cells are descended from cells from 2 fetuses whose mothers voluntarily decided to have legal abortions approx. 40 years ago, one in England, one in Sweden
• These descendent cells were never part of the aborted fetus
why are fetal cells used (summary)
fetal cells are used in the production of some vaccines. have since been used to create vaccines such as hepatitis A, chickenpox, rubella, and one rabies vaccine. No new abortions have been performed for vaccine development since then. Some vaccines may contain trace amounts of DNA from those original cells.
The Catholic Church has addressed this issue. Although it opposes abortion, it is acceptable—and even important—for Catholics to receive these vaccines, as they help protect health and save lives.
influenza risk factors for serious complications
• Age (under 2 yrs & over 65 yrs)
• Living in nursing home/long term care facility
• Underlying health conditions: • Asthma • Chronic lung disease • Blood disorders (e.g., sickle cell disease) • Diabetes • Heart disease • Kidney disorders • Liver disorders • BMI > 40
• Weakened immune system
• Pregnant people & up to 2 weeks after end of pregnancy
influenza transmission
mainly by inhaling airborne droplets when infected people cough, sneeze, or talk
less often by touching a contaminated surface and touching your own mouth, nose or eyes
4 types of influenza viruses
• Influenza A and B viruses – cause seasonal flu
• A new Influenza A can cause a pandemic when it: • It infects humans and causes illness • It spreads easily and sustainably (continues without interruption) among humans • Is different enough so people have little to no immunity to it
• Influenza C viruses – generally mild illness
• Influenza D viruses – mostly affect cattle; not known to cause illness in humans
Pandemic Flu Risk with Influenza A viruses
• broad host range including birds, humans, other mammalian hosts
• potential for zoonotic disease
• difficult to control spread of diseases in wild animals
• Reassortment
Reassortment
• when two influenza viruses infect the same host cell at the same time and exchange genetic material – this can create a novel influenza virus
• Reassortment strains produced the influenza pandemics of 1957, 1968 and 2009
Avian (bird) Influenza A Reassortment
Bird flu – hard to control because it can be carried long distances & transmitted by wild birds
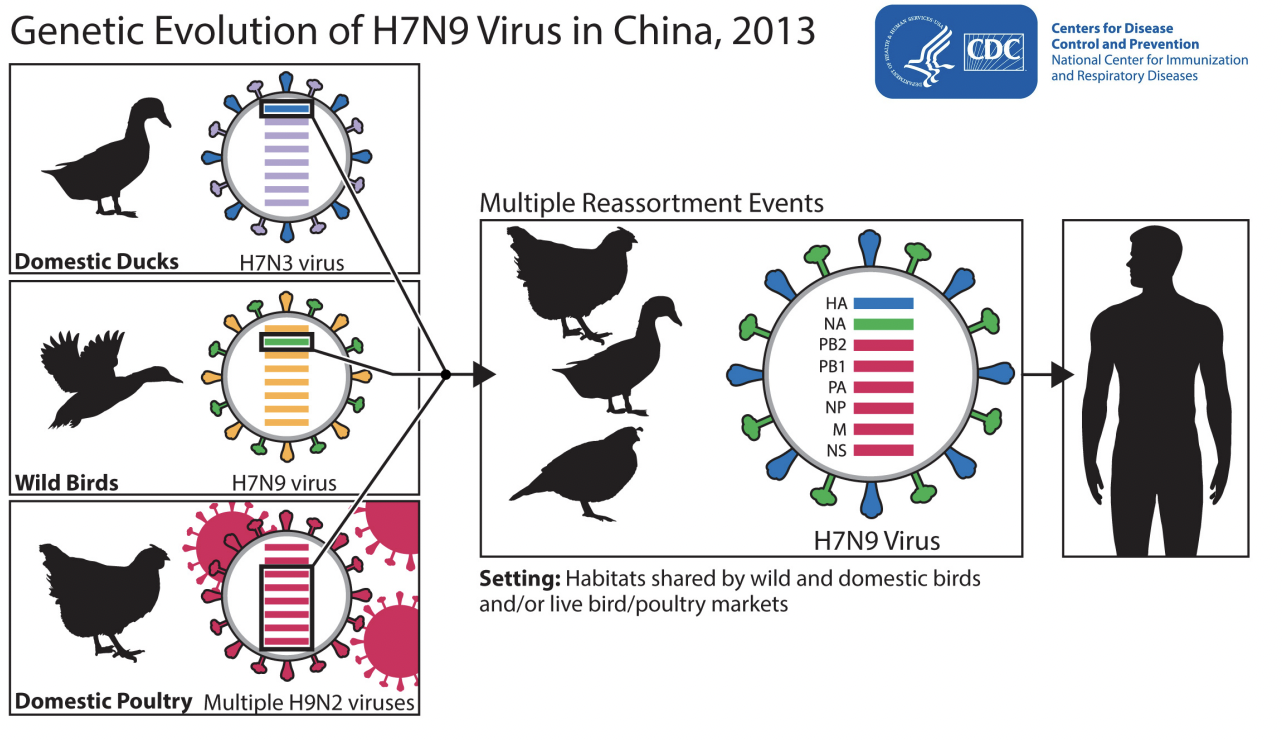
Viral host jump: disease transmission to new species (summary)
At a Maryland fair in 2017, pigs showed signs of illness, and 40 people contracted swine flu—highlighting a case of a virus jumping from animals to humans. These cross-species infections, or host jumps, are rare but potentially dangerous, sometimes leading to epidemics.
Viruses infect hosts in three steps: contact, infection/replication, and transmission. To infect a new species, a virus must overcome genetic differences and immune defenses. Most attempts fail, but viruses mutate rapidly, increasing the chance of successful adaptation—especially if the new host is genetically similar to the original one.
A virus that manages to replicate and transmit in a new species becomes especially dangerous, with each new host increasing the chance of mutations that enhance its spread. Though predicting epidemics is hard due to the diversity of viruses, researchers monitor viruses and mutations to better prepare for and contain future outbreaks.
Pandemic Influenza
Conditions that initiate a flu pandemic:
• new influenza virus emerges (little/no human immunity)
• It infects humans and causes illness
• It spreads easily and sustainably (continues without interruption) among humans
Strategies to address infections in afghanistan polio
In 2024, the World Health Organization (WHO) and the United Nations Children's Fund (UNICEF), with the backing of the Taliban, initiated Afghanistan's first nationwide polio vaccination campaign in over three years. This effort aimed to immunize millions of children under five against polio, addressing the resurgence of the disease in the country.
Tracking Vaccination Coverage
To monitor who received the vaccines, health workers employed a house-to-house strategy, marking the fingers of vaccinated children with indelible ink. This method ensured accurate tracking and helped identify unvaccinated children in subsequent rounds. Additionally, data collection tools and community registers were used to record vaccination details, facilitating efficient monitoring and follow-up.
Maintaining the Cold Chain
The "cold chain" refers to the temperature-controlled supply chain essential for preserving vaccine potency from manufacture to administration. In Afghanistan, maintaining this chain involved:
Cold Storage Facilities: Provincial vaccine storage centers, like the one in Farah, were equipped to store up to 1 million vaccine doses, ensuring a steady supply for both routine immunizations and campaigns. citeturn0search4
Refrigeration Equipment: Installation of cold rooms, refrigerators, and freezers at various health facilities ensured vaccines remained within the required temperature range.
Power Solutions: Given Afghanistan's unreliable electricity supply, backup generators and solar-powered systems were deployed to maintain consistent refrigeration.
Logistical and Operational Challenges
The campaign faced several hurdles:
Security Concerns: Health workers operated in areas with potential threats, necessitating coordination with local authorities and, at times, Taliban security forces to ensure their safety.
Geographical Barriers: Afghanistan's rugged terrain and remote villages posed transportation challenges, requiring the use of motorcycles, donkeys, or traveling on foot to reach certain areas.
Infrastructure Limitations: Poor road conditions and limited transportation infrastructure hindered timely vaccine delivery and movement of health personnel.
Coordination Among Stakeholders
Effective collaboration was crucial:
Engaging the Taliban: Securing the Taliban's support was pivotal. Their endorsement allowed access to previously unreachable areas and facilitated smoother operations.
Community Involvement: Local leaders and influencers were engaged to promote the campaign's importance, helping to build trust and encourage participation.
Inter-Agency Collaboration: WHO, UNICEF, and other partners coordinated efforts to ensure resource optimization, avoid duplication, and address challenges promptly.
Communication Strategies
To ensure public awareness and acceptance:
Mass Media Campaigns: Radio broadcasts, posters, and community announcements were used to disseminate information about the campaign's schedule and benefits.
Community Mobilizers: Trained individuals, including female health workers, engaged directly with families to educate them about the importance of vaccination, address concerns, and dispel myths.
Feedback Mechanisms: Channels were established for communities to voice concerns or report issues, allowing for real-time adjustments and increased trust in the campaign.
Despite the challenges, the concerted efforts of international organizations, local authorities, and communities marked a significant step toward eradicating polio in Afghanistan.
Strategies to address infections in nigeria polio
Based on the provided text about Nigeria’s Emergency Operations Centers (EOCs) and their role in eradicating polio and managing other disease outbreaks like COVID-19, here’s how they addressed the same key questions as before:
1. How did they track who got the vaccines?
Data-driven surveillance was central to Nigeria's polio eradication effort. EOCs analyzed real-time disease surveillance data to locate virus hotspots and track vaccination coverage.
Community health workers conducted door-to-door vaccination campaigns, ensuring coverage and identifying missed children.
EOCs allowed for central coordination of data collection, analysis, and response, enabling timely interventions.
2. What is cold chain and how did they maintain it?
Though not detailed in this text, the cold chain refers to the temperature-controlled supply system that keeps vaccines viable.
Nigeria's polio eradication involved a massive supply chain effort, including distribution from the national level down to remote communities, ensuring cold chain integrity through proper storage and transport infrastructure.
These same logistics systems were repurposed during the COVID-19 response, showing resilience and adaptability in maintaining cold chain needs.
3. What logistical or other challenges did workers experience?
Pre-EOC, Nigeria struggled with ongoing virus transmission and lacked the organizational infrastructure to contain outbreaks effectively.
Implementing the EOCs required mobilizing financial, human, and technical resources, which was a significant challenge initially.
Coordinating vast networks of health workers, community mobilizers, data analysts, and logistics personnel posed logistical complexity but became a strength of the EOC model.
4. What were the challenges in coordinating between all interested parties, including the government?
Before EOCs, efforts were fragmented. The EOC model brought government agencies, global partners, technical experts, and communities together under a unified strategy.
Coordination involved negotiations with local and religious leaders, critical to gaining public trust and participation.
The model promoted transparent decision-making, data sharing, and strategic alignment among stakeholders.
5. What were the challenges related to infrastructure and transportation?
Delivering vaccines across Nigeria’s vast and sometimes inaccessible regions required overcoming transportation and cold storage challenges.
The EOCs helped optimize logistics by ensuring vaccines and materials reached frontline workers, even in remote areas.
Infrastructure weaknesses—like poor roads or limited electricity—required innovative, localized solutions.
6. How did they work effectively with communication?
Communication was a cornerstone of success. The EOC engaged community and religious leaders, who were trusted voices in encouraging vaccination.
Teams focused on social mobilization, involving health workers with deep local knowledge to dispel misinformation and increase vaccine acceptance.
Consistent messaging and community engagement were key, especially during outbreaks like Ebola and COVID-19, leveraging lessons learned from polio.
Conclusion:
Nigeria’s Emergency Operations Centers proved vital in eradicating polio and managing new health crises like Ebola and COVID-19. Their strength lay in centralized coordination, data-driven response, strong logistics, and community-based communication—all of which offer a robust model for future pandemic preparedness.
Strategies to address infections in a variety of nations - UK public health rapid support team
Based on the text profiling the UK Public Health Rapid Support Team (RST), supported by Bill Gates, here’s how they addressed the same set of questions as before — regarding their response to disease outbreaks globally:
1. How did they track who got the vaccines or was exposed?
Surveillance was the cornerstone of RST operations. The team acted as disease detectives, conducting contact tracing to identify where infected individuals had been and who they may have exposed.
This involved meticulous tracking of patient movements—restaurants, taxis, healthcare facilities—and following up with potentially exposed contacts to prevent further spread.
While the team focused more on containment and diagnostics rather than mass vaccination, tracking and monitoring of cases was central to their strategy.
2. What is cold chain and how did they maintain it?
Although this specific text doesn't detail cold chain logistics, it's implied that diagnostic and laboratory capabilities were critical, and setting up field labs (e.g., in Sierra Leone) would have involved managing equipment that often requires temperature control.
Given the urgency and mobility of the RST, it’s likely they coordinated with organizations like WHO or local health ministries to ensure supplies, possibly including temperature-sensitive materials, were preserved properly en route and on site.
3. What logistical or other challenges did workers experience?
Speed of deployment was a core challenge — the RST aimed to be on the ground within 48 hours of an outbreak, which required rapid mobilization of people, supplies, and information.
Operating in low-resource environments often meant setting up or improving local healthcare infrastructure (e.g., building diagnostic labs).
RST worked during natural disasters (like the Sierra Leone mudslide), which further complicated logistics due to damaged infrastructure and displaced populations.
4. What were the challenges in coordinating between all interested parties, including the government?
The team did not act independently, but in collaboration with national governments, the World Health Organization, and local healthcare professionals.
A key challenge was to offer expertise without imposing control — their mission was to support local capacity rather than dominate response efforts.
They emphasized building trust and strengthening local systems to ensure sustainability and effectiveness in each country.
5. What were the challenges related to infrastructure and transportation?
Deploying to remote or crisis-stricken areas often involved weak infrastructure, including limited roads, power, water, or medical facilities.
In Sierra Leone, the RST had to establish a new lab from scratch within a hospital to provide diagnostics for cholera, salmonella, and dysentery.
Logistics for transporting equipment and safely deploying staff under such conditions posed significant challenges.
6. How did they work effectively with communication?
RST emphasized communication with local and national partners, integrating into existing outbreak response systems rather than operating in isolation.
They listened to local needs and avoided a “fly in, take over, and fly out” mentality, which helped foster collaboration and trust.
Public health messaging and coordination were likely handled in collaboration with WHO and local health ministries, ensuring culturally sensitive and effective outreach.
Conclusion:
The UK Public Health Rapid Support Team is a nimble, collaborative, and highly skilled group designed to respond rapidly to global disease outbreaks. Through swift deployment, data-driven surveillance, partnerships with local authorities, and infrastructure support, they aim not only to stop current outbreaks but to strengthen global public health systems — ensuring the world is better prepared for the next pandemic.
Strategies to address infections in a variety of nations - dem repub of congo ebola
Here’s an analysis of the Ebola outbreak response in the Democratic Republic of Congo (DRC) as described in the provided text, using the same questions we've applied in previous prompts:
1. How did they track who got the vaccines or was exposed?
Checkpoint systems were set up across the region to monitor symptoms, especially fever, which is a key indicator of Ebola. People were screened as they traveled, helping to isolate potential cases before further transmission.
However, many individuals sought care from traditional healers or local clinics first, making it difficult to identify and track all exposed individuals in time.
Mistrust and reluctance to visit Ebola treatment centers also meant some exposed individuals were never documented or reached in time for effective intervention.
2. What is cold chain and how did they maintain it?
The text doesn’t detail cold chain logistics, but the deployment of the Ebola vaccine, which requires strict cold storage, implies that a cold chain infrastructure was in place and maintained by international health organizations like the WHO and partners.
Given the instability in the region, maintaining cold chain would have been a logistical challenge, likely involving portable refrigeration units and secure supply routes.
3. What logistical or other challenges did workers experience?
Security was a major issue. Ebola centers were attacked and burned, requiring military protection for responders and facilities.
Workers had to operate in conflict zones, which increased risk, delayed efforts, and fueled public mistrust.
Ebola response teams, dressed in protective suits and using chlorine to disinfect belongings, were seen as frightening or alien, contributing to resistance from local communities.
4. What were the challenges in coordinating between all interested parties, including the government?
The complicated political landscape and history of conflict in the DRC made coordination between international organizations, the national government, and local communities extremely difficult.
Some communities were suspicious of government and international responders, making public health coordination more complex.
WHO leadership (including Dr. Tedros) was personally involved in trying to build understanding and support on the ground, underscoring how tense and delicate the coordination process was.
5. What were the challenges related to infrastructure and transportation?
Many Ebola treatment centers were hours away from affected communities. This discouraged families from sending loved ones to get treatment.
The spread of Ebola to large cities like Goma, hundreds of kilometers from initial outbreak areas, highlighted weaknesses in infrastructure and surveillance, allowing infected individuals to travel across regions undetected.
Local health facilities were overwhelmed, often lacking the capacity to safely isolate or treat suspected cases.
6. How did they work effectively with communication?
Communication was a major challenge, especially due to mistrust, fear, and cultural differences.
Responders had to navigate the emotional and cultural dimensions of the crisis — for instance, a mother refusing to send her child to a distant Ebola center due to fear and maternal instinct.
Visuals such as “plastic cubes” in treatment centers were introduced to allow families to see and communicate with patients, improving transparency and trust.
Public health messaging competed with traditional belief systems, requiring deep community engagement to be effective.
Conclusion:
The Ebola response in the DRC, though strengthened by improved treatments and vaccines, was deeply hindered by conflict, weak infrastructure, public mistrust, and logistical challenges. The situation highlights how health interventions must be as socially and politically strategic as they are medically advanced, and that trust-building, local engagement, and long-term investment are key to containing outbreaks.