OCR A Level Chemistry - Chapter 6 Shapes of Molecules and Intermolecular Forces
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
Describe the electron pair repulsion theory
Electron pairs repel each other as far as possible due to negative charge. The arrangement of electrons minimises repulsion, holding bonds in a definite shape.
What determines the shape of a molecule?
The electron pairs surrounding a central atom
What does a solid wedge represent?
a bond that comes out of the plan of the paper
What does a dotted wedge represent?
a bond that goes into the plan of the paper
Why do lone pairs repel more strongly than bonded pairs?
lone pair of electrons slightly closer to the central atom and occupies more space than a bonded pair
State the order of increasing repulsions between lone pairs and bonding pairs
bonded pair/bonded pair < bonded pair/lone pair < lone pair/lone pair
How much is the bond angle reduced by for each lone pair?
2.5
What is meant by the term 'electron density'?
the probability of an electron being present at a specific location
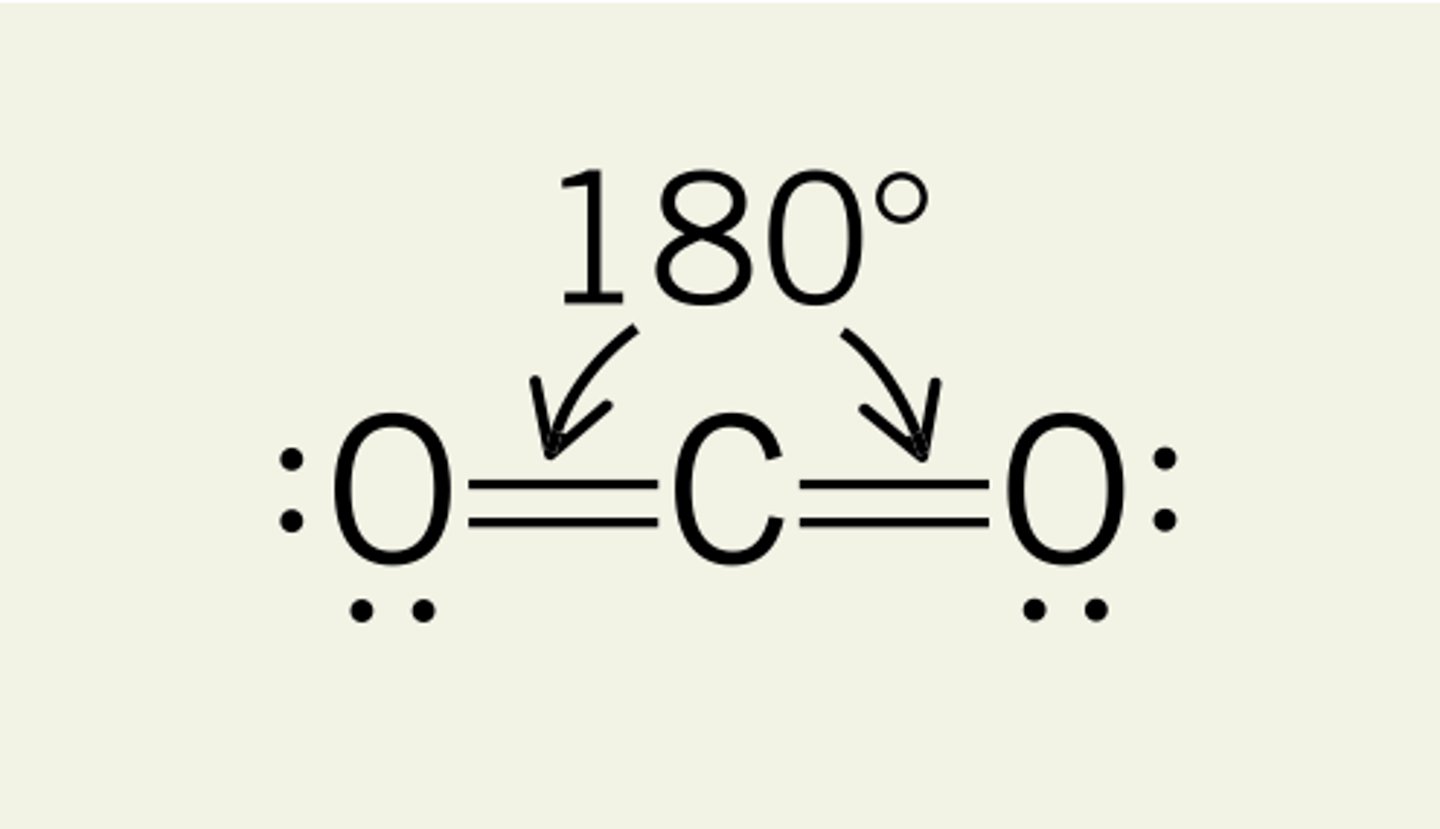
What is the shape and bond angle of a molecule with 2 bonded pairs of electrons?
-linear
-180
-e.g. CO2
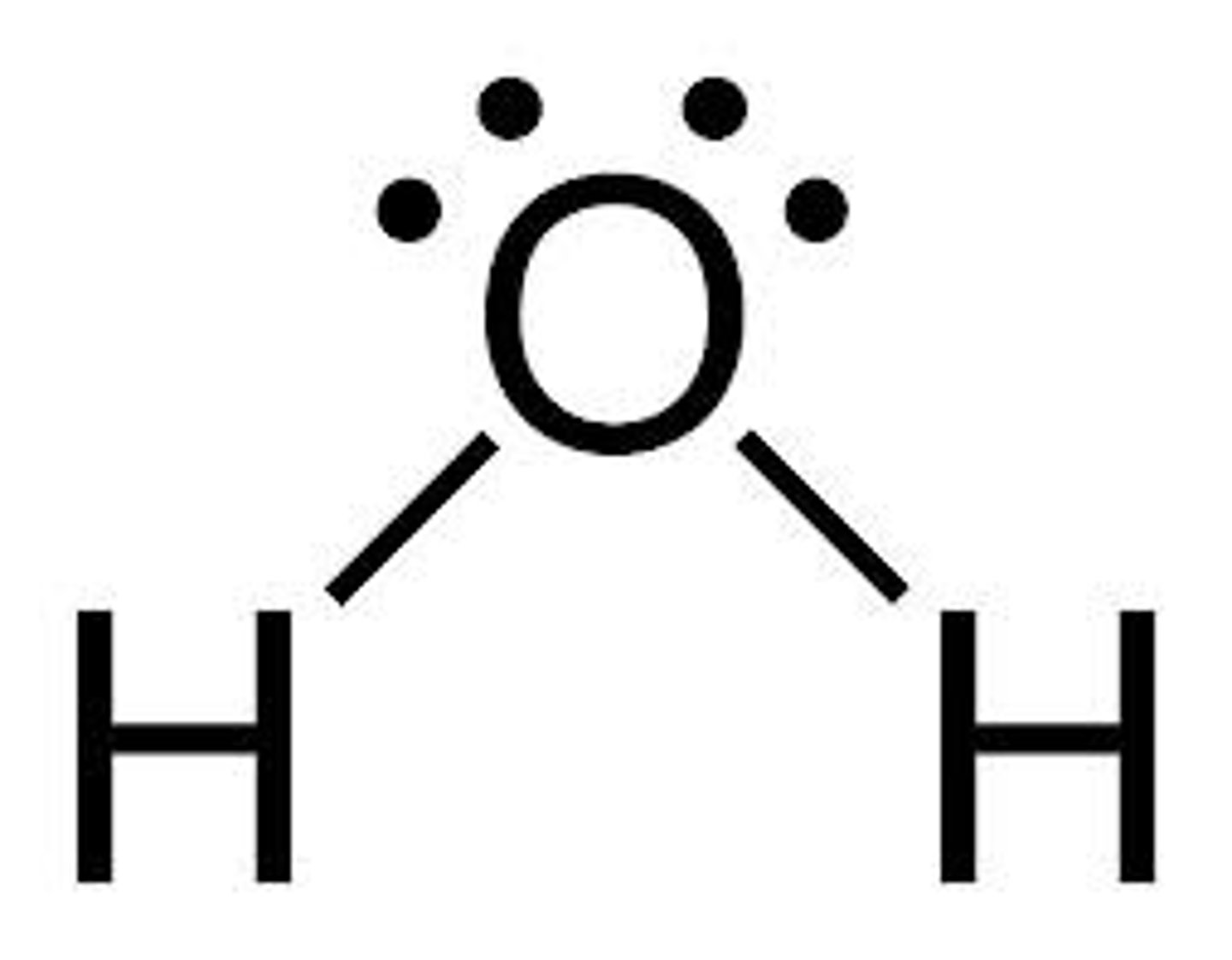
What is the shape and bond angle of a molecule with 2 bonded pairs and 2 lone pairs?
-non-linear
-104.5
-e.g. H2O
What is the shape and bond angle of a molecule with 3 bonded pairs of electrons?
-trigonal planar
-120
-e.g. BCl3

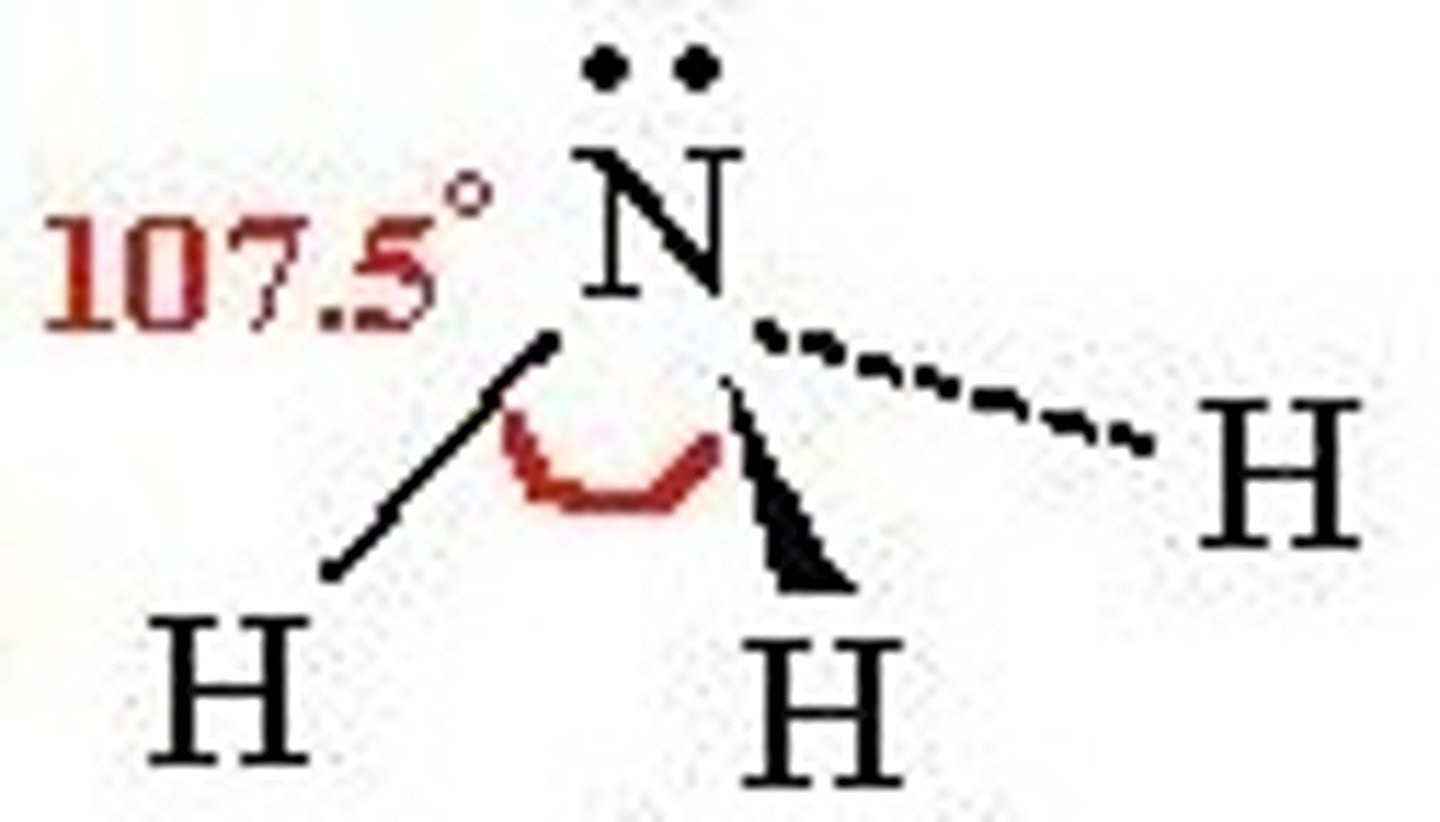
What is the shape and bond angle of a molecule with 3 bonded pairs and 1 lone pair?
-pyramidal
-107
-e.g. NH3
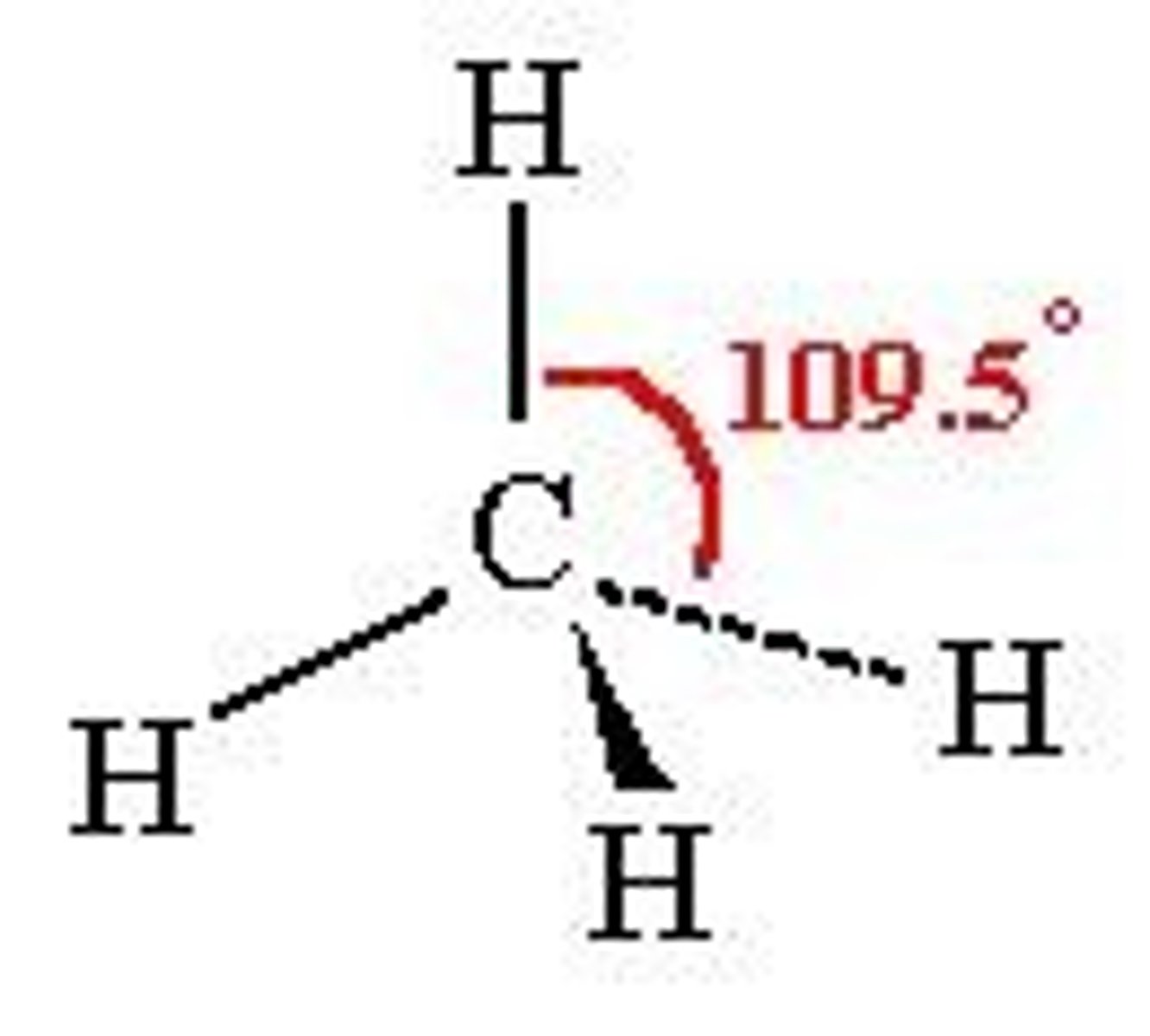
What is the shape and bond angle of a molecule with 4 bonded pairs of electrons?
-tetrahedral
-109.5
-e.g. CH4
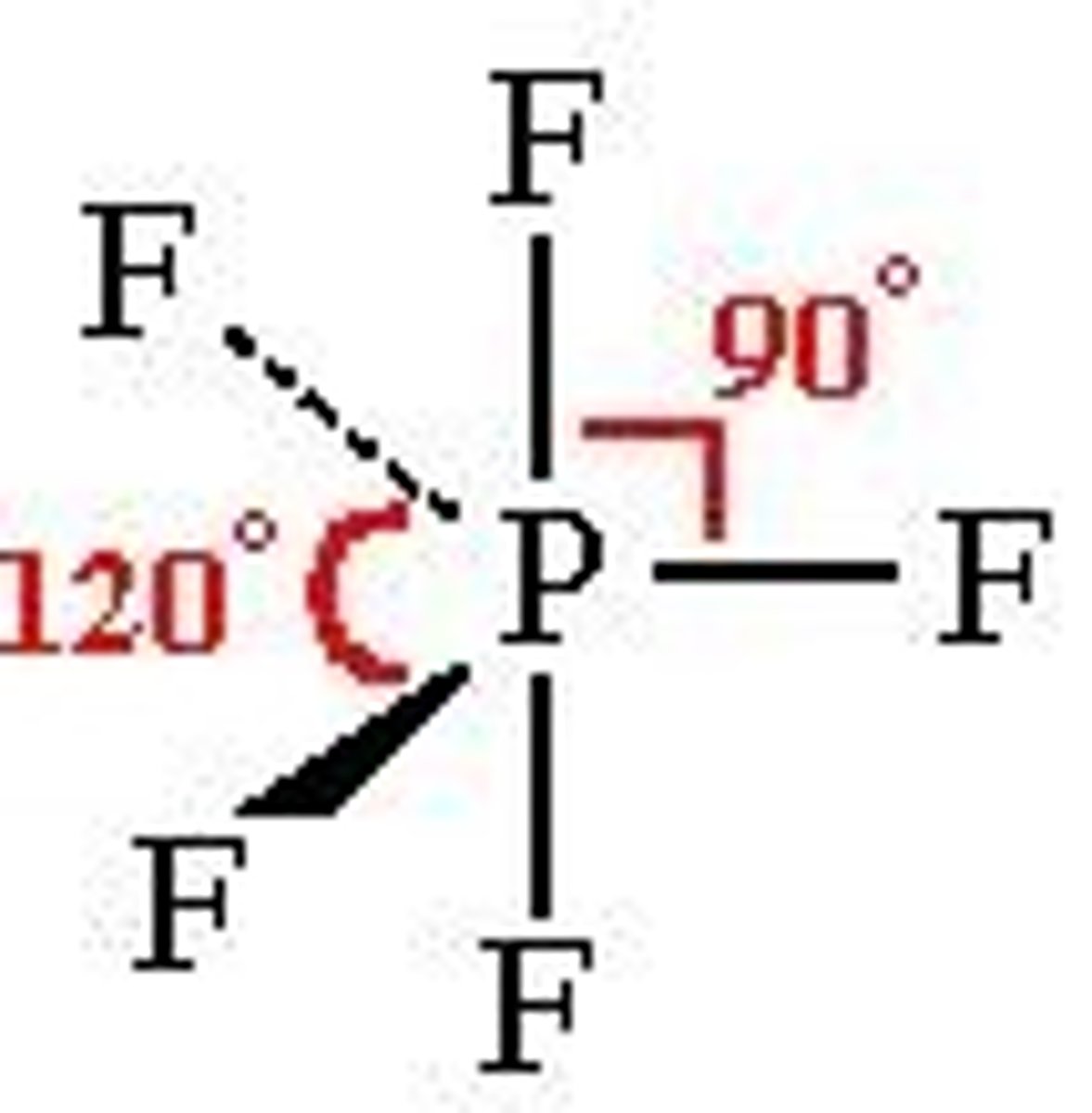
What is the shape and bond angle of a molecule with 5 bonded pairs of electrons?
-trigonal bipyramidal
-90 and 120
-e.g. PCl5
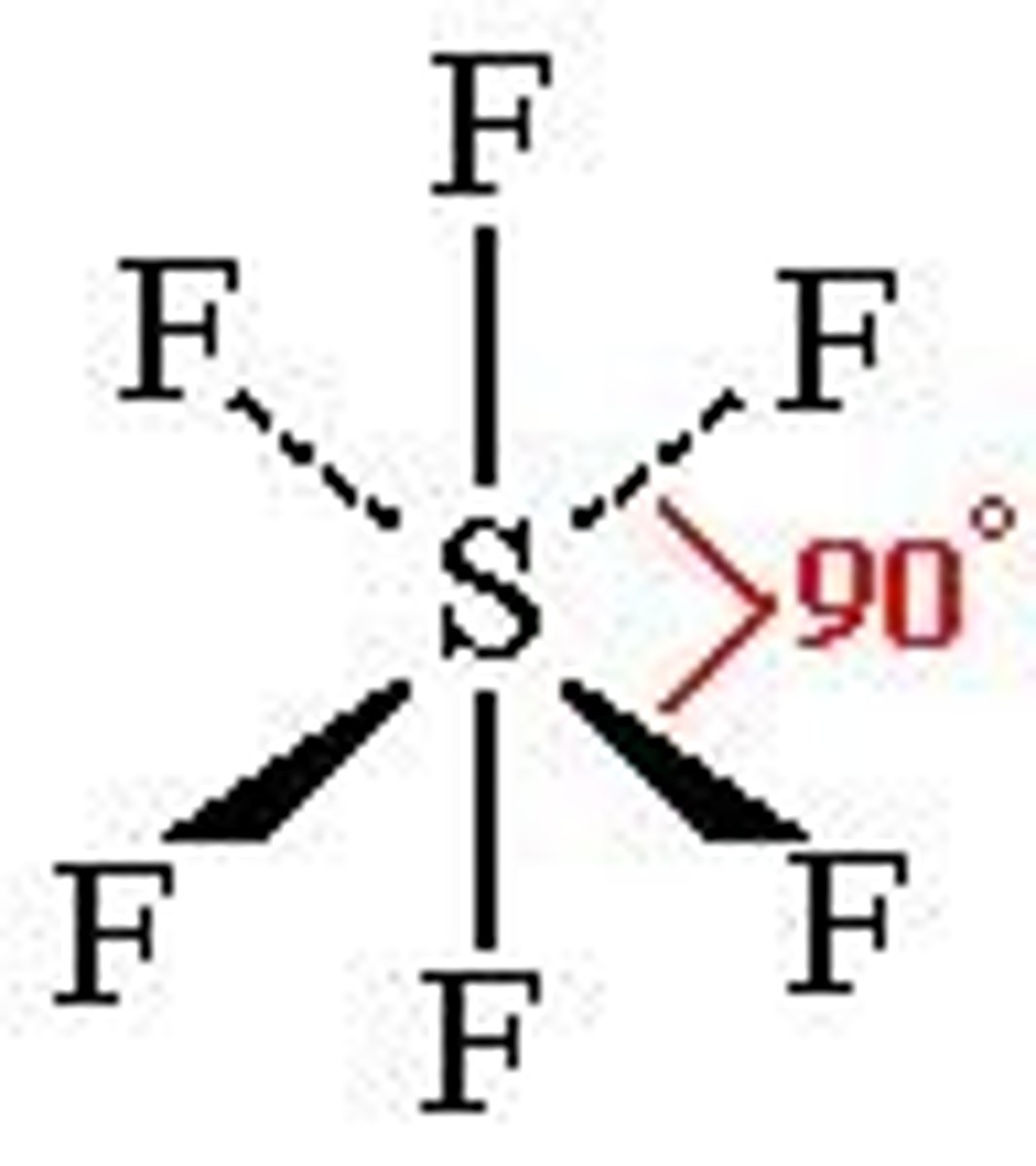
What is the shape and bond angle of a molecule with 6 bonded pairs of electrons?
-octahedral
-90
-e.g. SF6
What is the shape and bond angle of a molecule with 2 bonded pairs and 1 lone pair?
-non-linear
-120
-SO2
What is electronegativity?
The ability of an atom to attract the bonding electrons in a covalent bond
When is the pair of electrons in a covalent bond equally shared?
when the bonded atoms are the same or have the same electronegativity
When is the pair of electrons in a covalent bond unequally shared?
when the bonded atoms are different and have different electronegativities
What is the Pauling scale used for?
to compare different electronegativities
Why are the pair of electrons in a covalent bond sometimes unequally shared?
-nuclear charges are different
-atoms may be different sizes
-the shared pair of electrons may be closer to one nucleus than the other
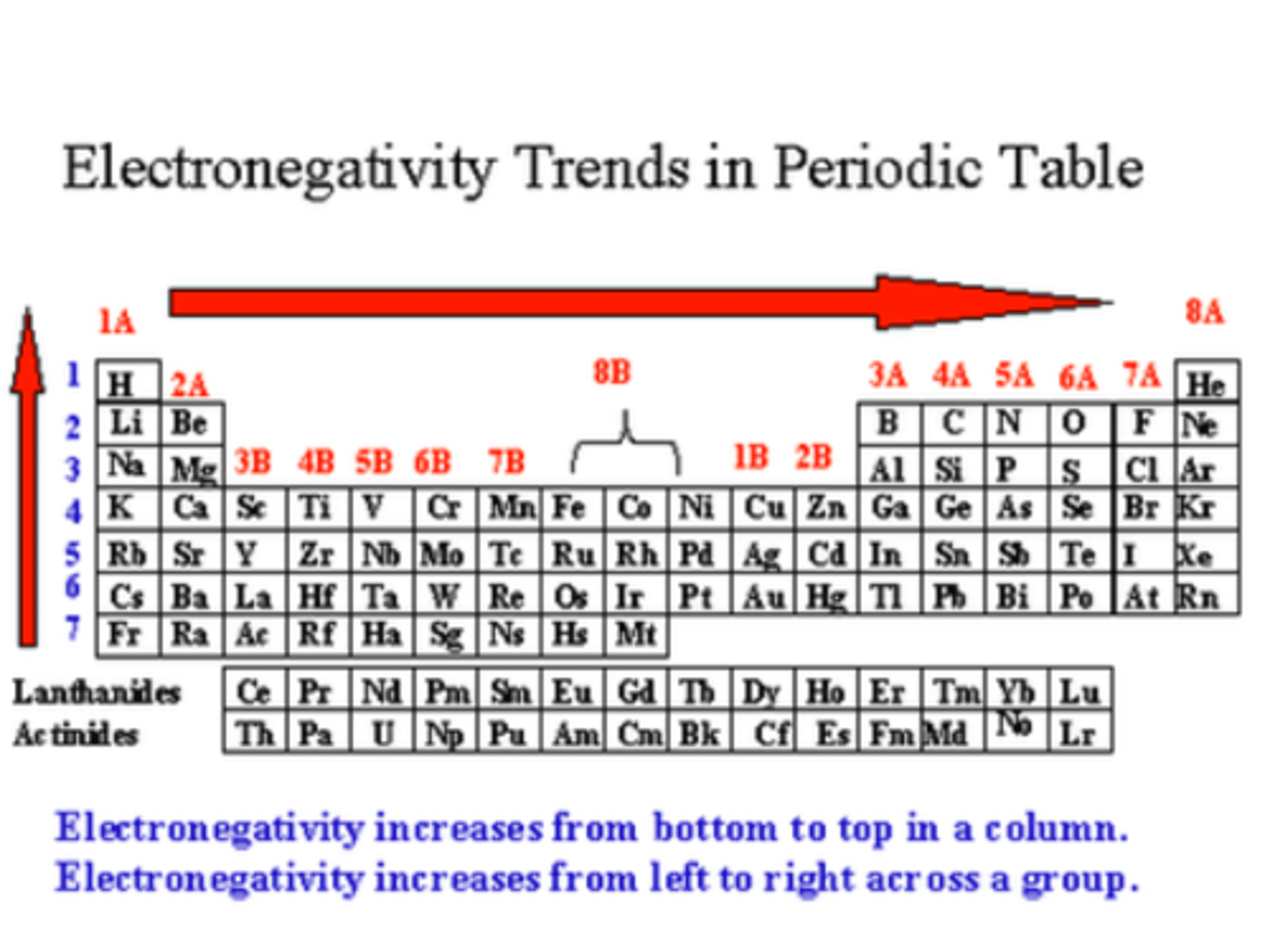
Describe the trend in electronegativity on the periodic table
electronegativity increases across and up the periodic table, with the most electronegative atom being fluorine
Why do noble gases not have Pauling numbers?
they don't form compounds
What is a non-polar bond?
a covalent bond in which electrons are shared equally
What is a pure covalent bond?
When the difference in electronegativity between atoms is 0
What is a polar bond?
a covalent bond in which electrons are shared unequally
What is the charge of the more electronegative element in a polar covalent bond?
partial negative charge

What is the charge of the less electronegative element in a polar covalent bond?
partial positive charge
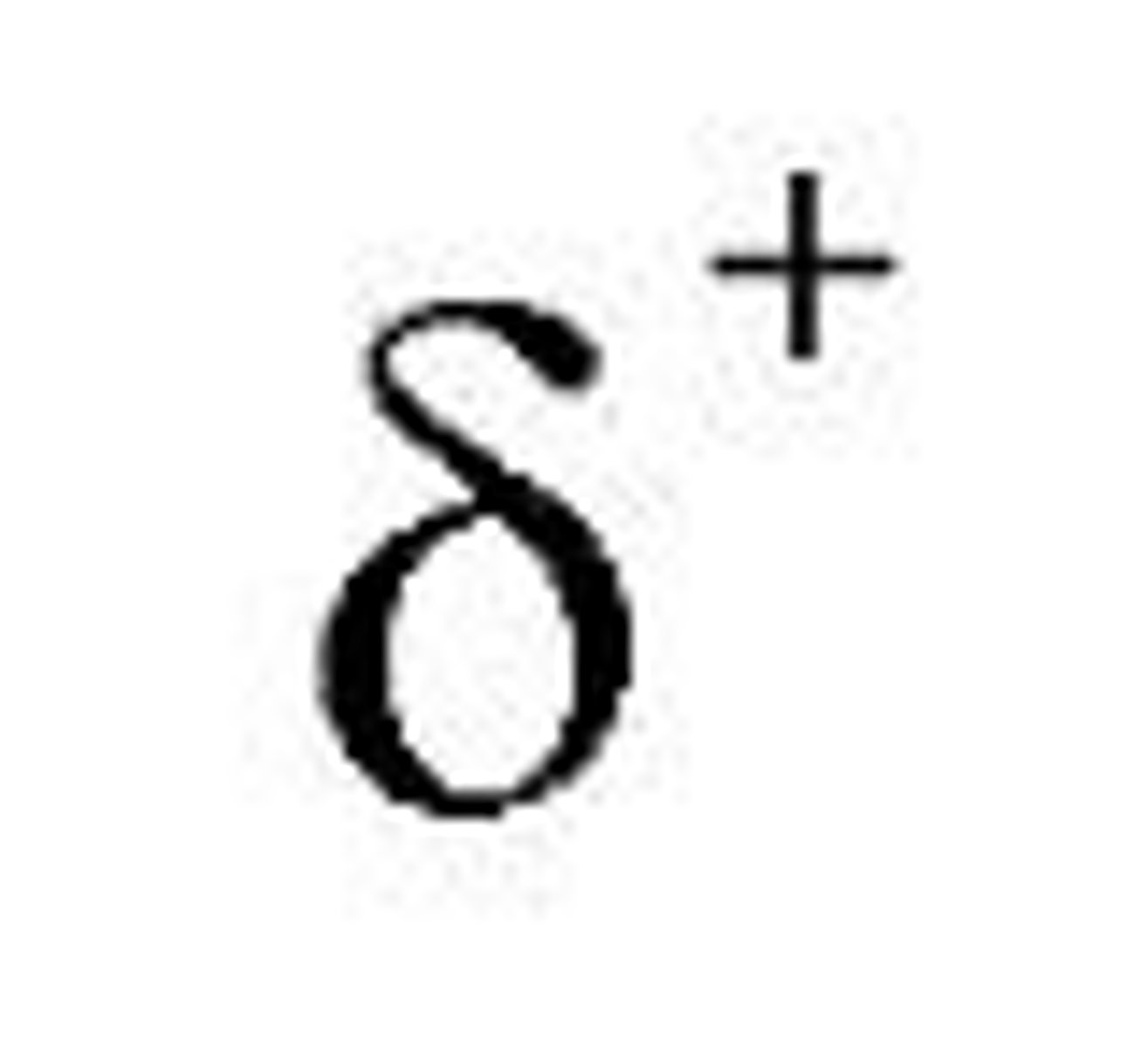
What is a dipole?
A molecule that has two poles
What is a permanent dipole?
the separation in partial charges across a polar bond, arising from different electronegativities
When can dipoles cancel out?
if the molecule is symmetrical and the dipoles act in opposite directions
Describe what happens when an ionic compound dissolves in a polar solvent
e.g. Na+ ions are attracted to oxygen (delta negative) and Cl- ions are attracted to hydrogen (delta positive)
-the ionic lattice breaks down as it dissolves
-in the resulting solution, the water molecules surround the ions
Why is carbon dioxide considered non-polar?
-the 2 C=O bonds each have a permanent dipole
-2 dipoles act in opposite directions and exactly oppose one another as the molecule is symmetrical
-the dipoles cancel out and the overall dipole is zero
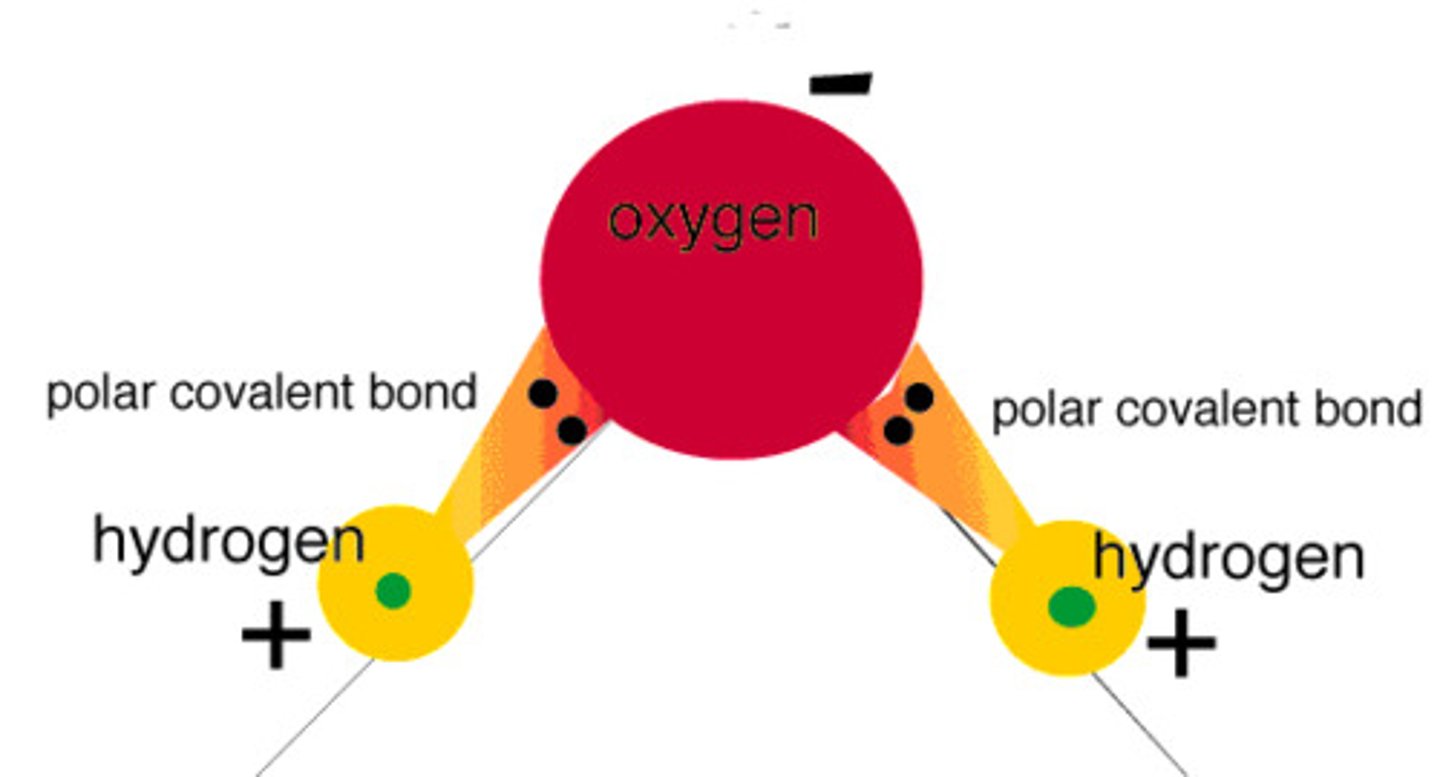
Why is water a polar molecule?
-2 O-H bonds each have a permanent dipole
-2 dipoles act in different directions but do not exactly oppose one another as the molecule is non-symmetrical
-overall, the oxygen end of the molecule has a partial negative charge and the hydrogen end has a partial positive charge
What are intermolecular forces?
Weak interactions between dipoles of different molecules
What are the 3 types of intermolecular forces?
-induced dipole-dipole interactions
-permanent dipole-dipole interactions
-hydrogen bonding
What are induced dipole-dipole interactions?
-the electrons in a molecule are constantly moving
-at any instant, an instantaneous dipole will exist
-the instantaneous dipole induces a dipole on a neighbouring molecule, which further induces a dipole on neighbouring molecules
-they attract one another
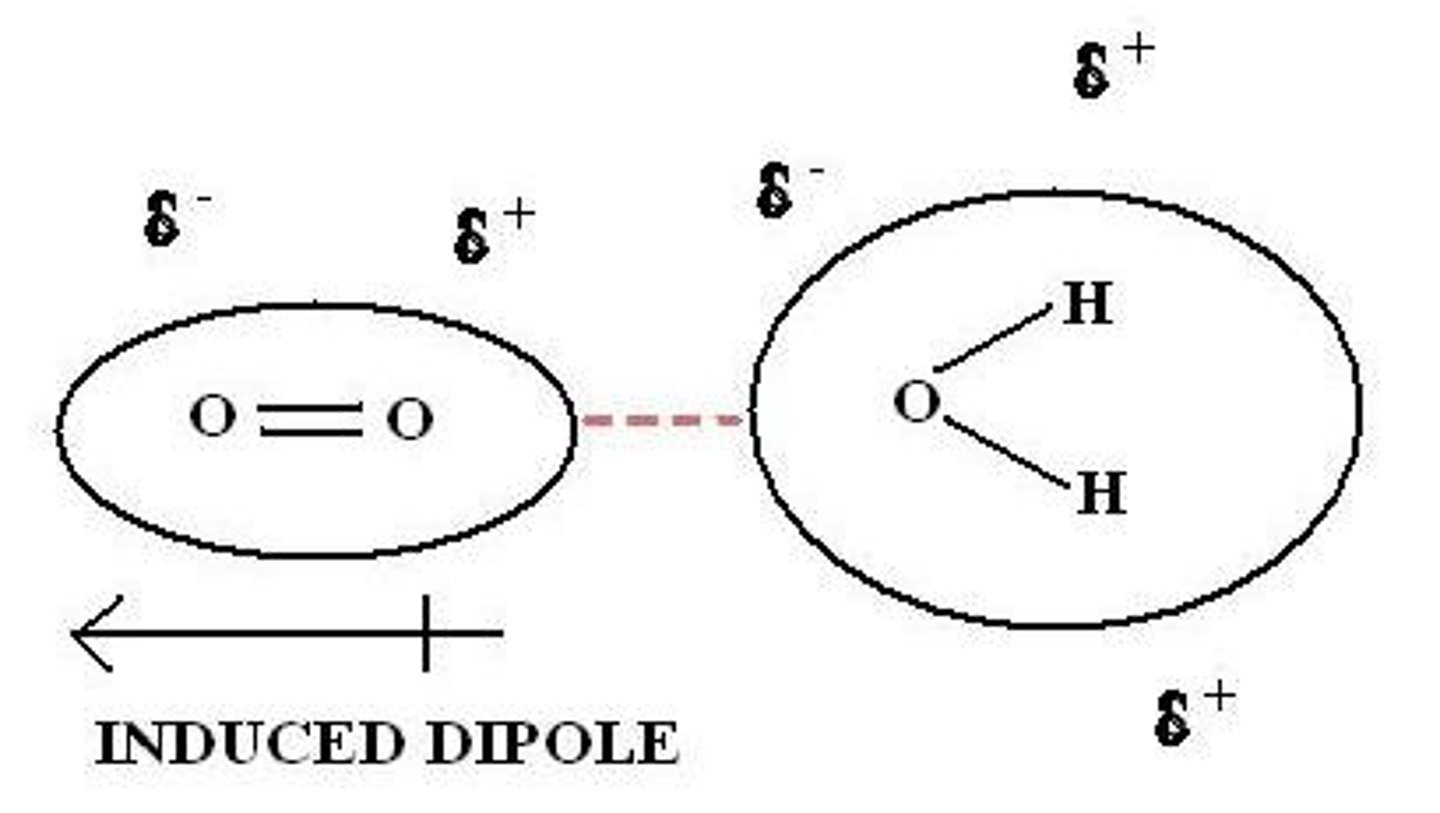
Where are induced dipole-dipole interactions found?
between all molecules
What is the strength of induced dipole-dipole interactions dependent on?
the electrons in a molecule
How does increasing the size of the molecule increase the boiling point?
more electrons present= larger instantaneous and induced dipoles= greater induced dipole-dipole interactions= stronger the attractive forces between molecules= more energy required to overcome forces
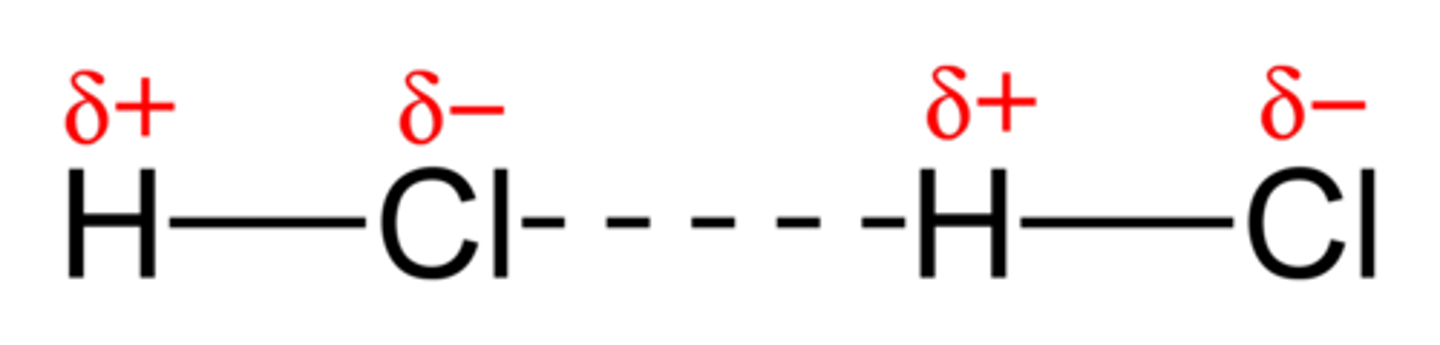
What are permanent dipole-dipole interactions?
The attraction between permanent dipoles in different polar molecules caused by difference in electronegativity of bonded atoms
What forces break when heated to give the boiling point?
intermolecular forces
What structure do simple molecular forces form when solid?
simple molecular lattice
What typical properties do simple molecular substances have?
-low melting and boiling point as the weak intermolecular forces are easily overcome by low temperatures
How does a non-polar simple molecule interact with a non-polar solvent?
-intermolecular forces form between molecules and solvent
-interactions weaken intermolecular forces in simple molecular lattice
-intermolecular forces break and the compound dissolves
Non-polar simple molecules tend to be soluble in non-polar solvents
How does a non-polar simple molecule interact with a polar solvent?
-there is little interaction between molecules
-intermolecular bonding in polar vent too strong to be broken
Non-polar simple molecular substances tend to be insoluble in polar solvents
How does a polar simple molecule interact with a polar solvent?
-polar covalent substances can dissolve in polar solvents as they attract each other
Solubility depends on strength of dipole
Can simple molecules conduct electricity?
no because there are no mobile charged molecules in simple molecular substances
What is a hydrogen bond?
a type of permanent dipole-dipole interaction found between a lone pair of electrons on an electronegative atom (e.g. O, N, F) and a hydrogen atom attached to an electronegative atom
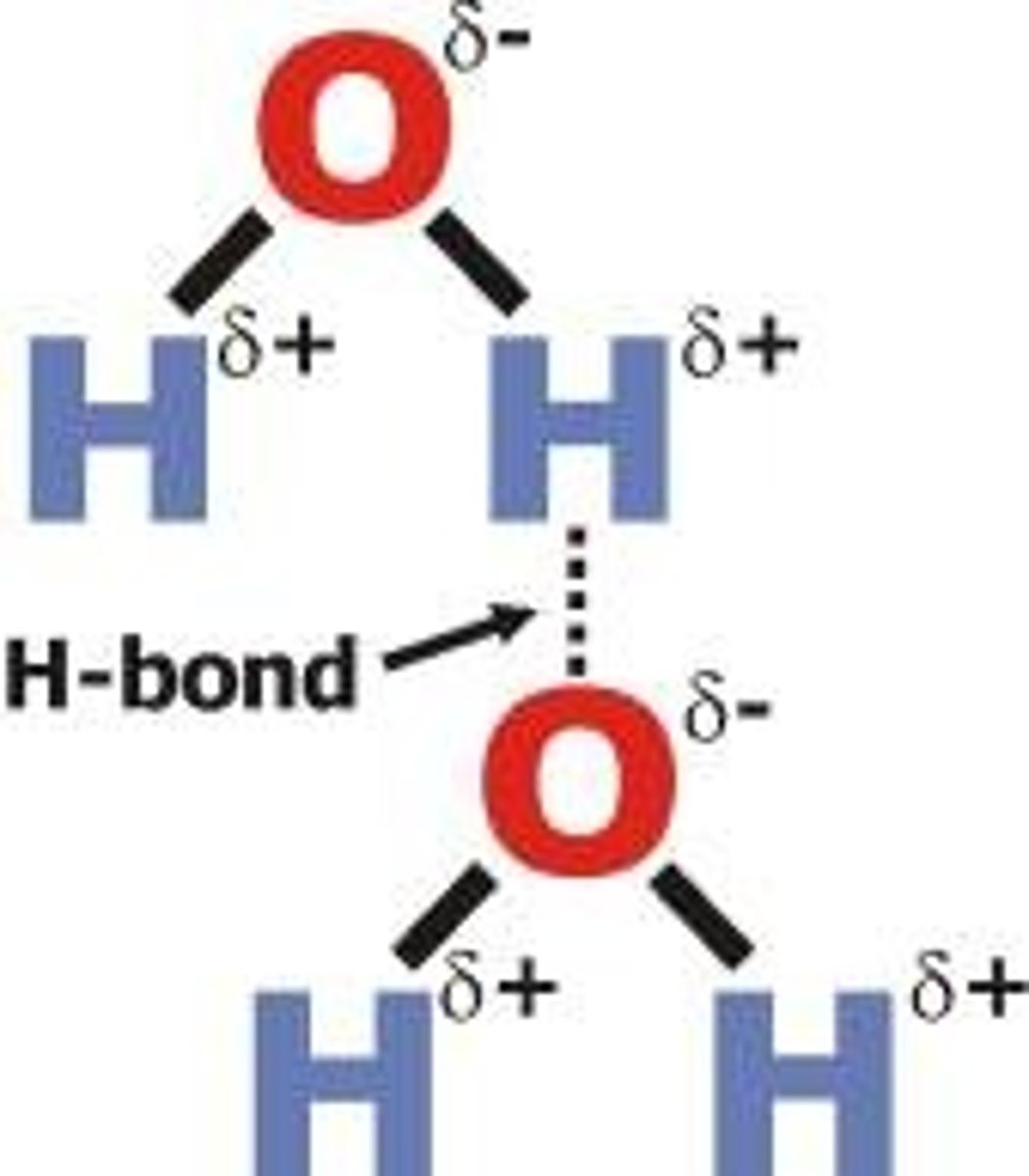
What is the strongest type of intermolecular attraction?
hydrogen bonding
Why is ice less dense than water?
in ice, the 4 hydrogen bonds in water form an open tetrahedral lattice full of holes, decreasing the density of water on freezing
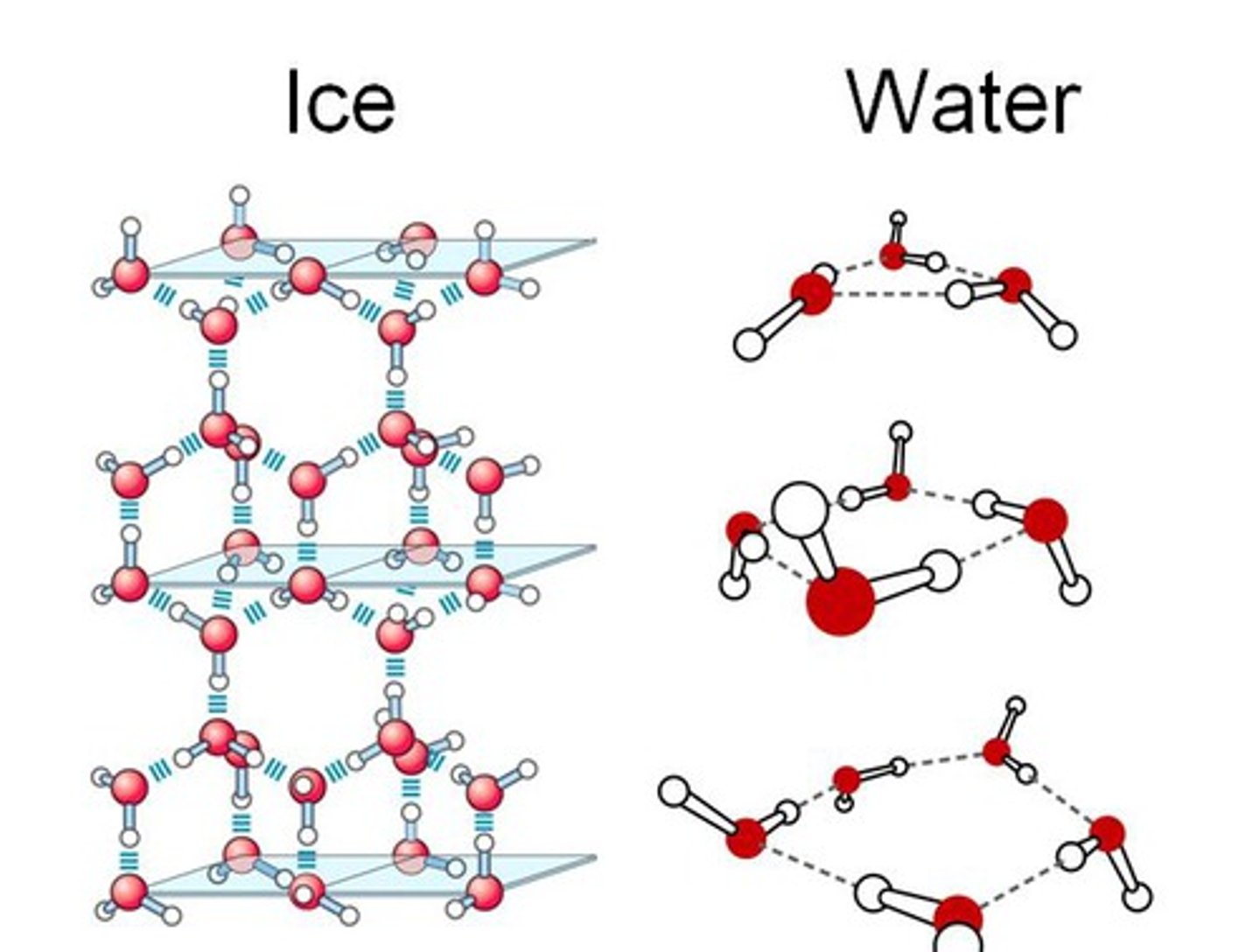
What happens when ice melts?
the open lattice collapses and the water molecules move closer together
Why has water got a higher melting and boiling point than expected?
as well as induced dipole-dipole interactions, hydrogen bonds occur. A high quantity of energy is needed to break these hydrogen bonds
Why can insects walk on water?
water has a high surface tension and viscosity
What is a polar covalent bond?
A molecule with an electronegativity difference between atoms between 0 and 1.8
How to determine an ionic bond from it's electronegativity difference
If it's electronegativity difference is greater than 1.8