Chromatography - 33
1/29
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Chromatography
An analytical technique that separates components in a mixture between a mobile phase & stationary phase
Mobile Phase
Liquid/Gas
Stationary Phase
Solid (TLC)
Liquid/Solid on a solid support (Gas chromatography)
Thin-Layer Chromatography
A plate is coated with a solid and a convent moves up the plate
Column Chromatography
A column is packed with a solid an a solvent moves down the column
Gas Chromatography
A column is packed with a solid/solid coated by a liquid & a gas is passed through the column under pressure at high temp
GC - Mobile phase
Inert gas - Nitrogen/Helium/Argon
GC - Stationary Phase
Liquid on an inert solid
Column Chromatography Separation
Dependent on the balance between solubility in the moving phase & retention in the stationary phase
Solid stationary phase separates by
Adsorption
Liquid Stationary phase separates by
Relative solubility
Identification
Retention times
Rf values
Polar Stationary Phase & Moving phase non polar
Non polar compounds would pass through the column faster that polar compounds (They’d have a greater solubility in the non polar moving phase)
TLC
a) Wearing gloves, draw a pencil line 1 cm above the bottom of a TLC plate and mark spots for each sample, equally spaced along line.
b) Use a capillary tube to add a tiny drop of each solution to a different spot and allow the plate to air dry.
c) Add solvent to a chamber or large beaker with a lid so that is no more than 1cm in depth
d) Place the TLC plate into the chamber, making sure that the level of the solvent is below the pencil line. Replace the lid to get a tight seal.
e) When the level of the solvent reaches about 1 cm from the top of the plate, remove the plate and mark the solvent level with a pencil. Allow the plate to dry in the fume cupboard.
f) Place the plate under a UV lamp in order to see the spots. Draw around them lightly in pencil.
g) Calculate the Rf values of the observed spots.
Justify:
Wear plastic gloves
Pencil line
Tiny drop
Depth of solvent
Lid
Solvent rising to the top
Dry in a fume cupboard
UV lamp
Wear plastic gloves - to prevent contamination from the hands to the plate
pencil line - will not dissolve in the solvent
tiny drop - too big a drop will cause different spots to merge
Depth of solvent - if the solvent is too deep it Will dissolve the sample spots from the plate
lid - to prevent evaporation of toxic solvent
Solvent reaching the top - Will get more accurate results if the solvent is allowed to rise to near the top of the plate but the Rf value can be calculated if the solvent front does not reach the top of the plate
Dry in a fume cupboard - the solvent is toxic
UV lamp used if the spots are colourless and not visible
Rf value
Distance moved by amino acid/distance moved by the solvent
Two Directional Chromatography (Two solvents are used)
Needed to separate a complex mixture where the components have similar solubilities in a solvent OR the mixture doesn’t separate with the first solvent
TDC Method
A spot of the mixture on a TLC plate is first separated with one solvent.
Then the TLC plate is rotated 90o and the plate is placed in a second solvent for a second
separation to take place
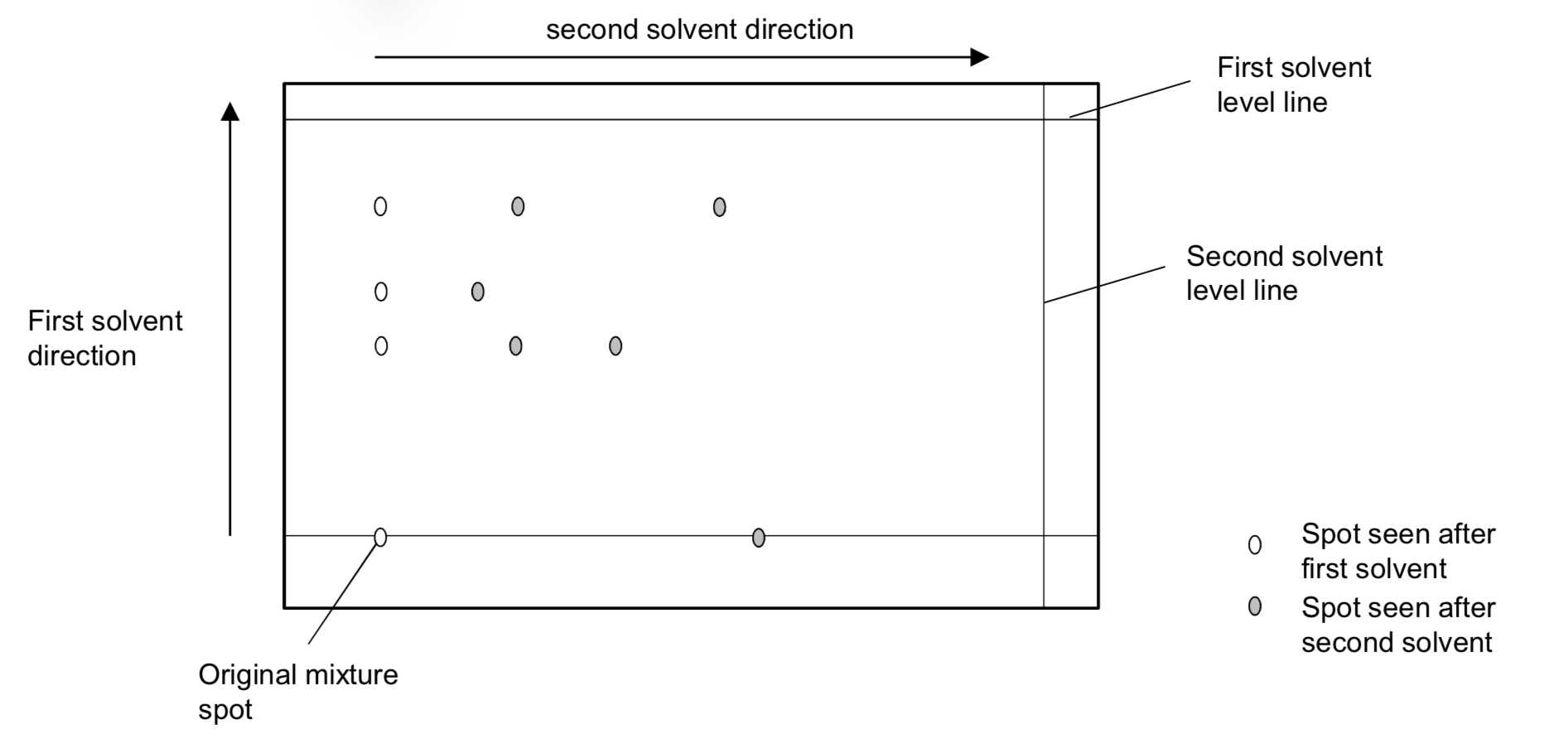
How many components are there
6
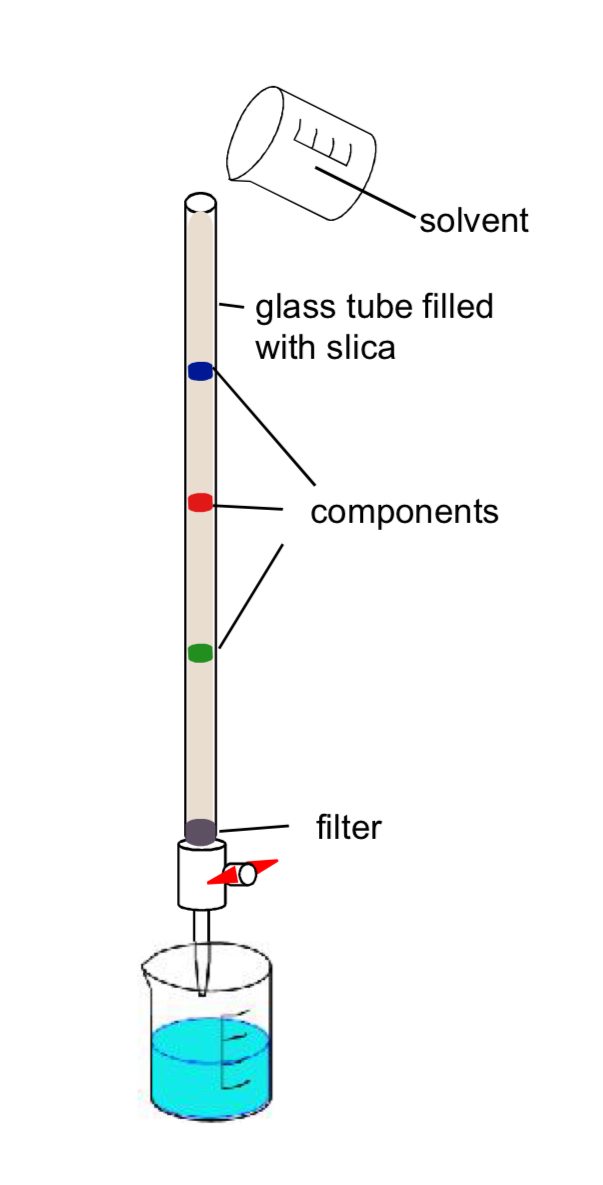
Simple Column Chromatography
• A glass tube is filled with the stationary phase usually silica or alumina in powder form to increase the surface area.
• A filter or plug is used to retain the solid in the tube. Solvent is added to cover all the powder.
• The mixture to be analyzed is dissolved in a minimum of a solvent and added to the column.
• A solvent or mixture of solvents is then run through the column.
• The time for each component in the mixture to reach the end of the column is recorded (retention time)
HPLC - High performance liquid chromatography
Industry used column chromatography
Solid silica = stationary phase
Liquid = Mobile phase
GC
Used to separate mixtures of volatile liquids
Retention Time
Time taken for a particular compound to travel from the injection of the sample to where it leaves the column to the detector
Basic GC
Shows how many components they are in the number of peaks + the abundance of each substance (area under the peak is proportional to the abundance)
Inert Carrier Gas
Will not react with the components separated in the GC column
Factors affecting retention times in GC
GC column temperature, column length , flow rate.
If the temperature or the flow rate is higher then substance will move more quickly through the column to give shorter retention times.
Connection to IR/NMR/Mass spec
GC machine can be connected to any of the above = all components to be identified
Similar retention times In GC
Components wouldn’t be distinguished
GC+MS
Used in Analysis
Forensics
Environmental Analysis
Airport Security
Space probes
Explain why each amino acid has different Rf values
Each amino acid has different relative affinity in stationary and mobile phases