Chemistry Safety
1/18
Earn XP
Description and Tags
Learning objectives At the end of this simulation, you will be able to: Understand how to create biodiesel from algal oil Identify the hazards posed by chemicals and how to handle them React quickly and save lives in case of a fire emergency Use the CAS numbers to plan your experiment Understand how to dispose of halogenated and non-halogenated waste Lookup H and P phrases in the safety data sheet Safely use a chemical fume hood
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
19 Terms
Click of the symbol that depicts explosive chemicals
Exploding Bomb

Which one symbolizes an oxidizing reagent
Flame over circle

Which symbol would be found on a bottle containing strong acid or base?
Corrosion
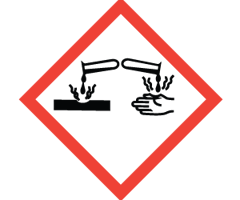
Which symbol indicates that this chemical shall not leak into the waterways?
Environment

How would you write the conversion reaction from oil to biodiesel?
Oil + alcohol + catalyst -> biodiesel -> glycerol -> catalyst
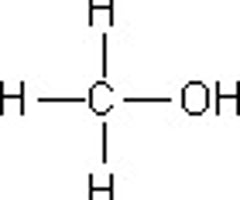
Which of the following does not apply for methanol?
(wear gloves and avoid in toxic vapors)
Health Hazard
Flammable
Toxic
Oxidizing
Oxidizing
Have a look a the Hazard identification section by clicking the 'view Therory' button Which P-sentence indicates why you should work in the hood when using methanol?
P260 to P271
Methanol is volatile because it has a low boiling point. Have a look at the safety data sheet. What is the boiling point?
65° C
Sodium Hydroxide
NaOH
CAS No. 1310-73-2
substance type: Mono-constituent
Why is it important to work in the film who during this step?
Methanol gets very vilatile at high temperature
There is more remaining sodium hydroxide-methanol solution that we have to dispose of.
This solution possesses several hacks, which of the following does not apply for the sodium hydroxide-methanol solution?
Explosive
(The sodium hydroxide methanol solution is flammable, toxic and corrosive)
Which of these hazards could be removed by adding small amounts of certain solution?
Corrosiveness
(If we add enough weak acid to the solution, we cannot realize the strongly basic solution)
Weak Acid
Acetic acid is a weak acid. It's main component of vinegar
How should do this post of the remaining methanol solution?
Into organic solvent waste
Imagine the two following scenarios
you pour a small amount of non-halogenated solvent Into the halogenated waste
You pour a small amount of halogenated solvent into the non-halogenated waste.
Which them in analysis is right
Is worse than 1.
Which of the following solvents would be disposed in the halogenated solvent waste?
Dichloromethane
I pass out because of the blast. What happened after I was pulled out of the lab?
Triggered the alarm and break the circuit
Why do you decide not to grab the fire extinguisher and fight the fire?
To dangerous
You are a true safety expert. Do you remember the hazards symbol for oxidizing reagents?
A circle with flames