KBKF15 - WEEK 7 - CHOLESTEROL AND INTEGRATION OF METABOLISM
1/23
Earn XP
Description and Tags
CHAPTER 26 & 27
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
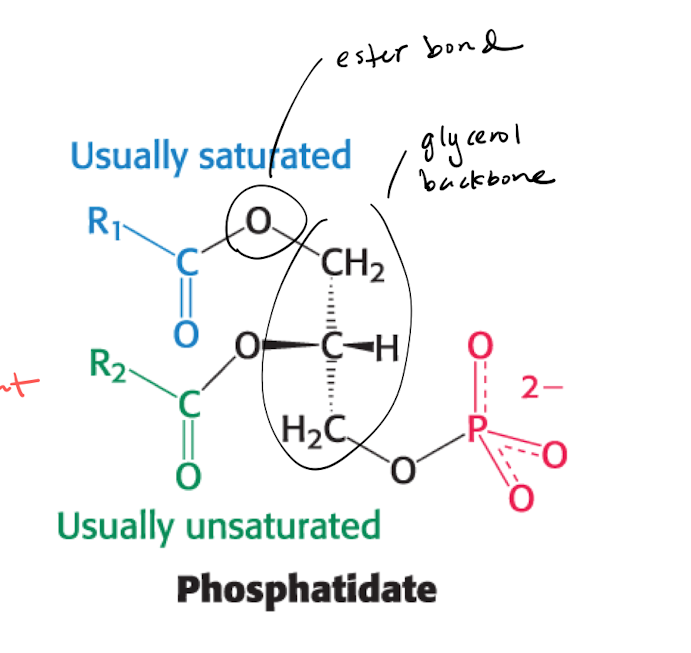
Phosphatide (PA) : why is it important?
Key intermediate for phospholipids and triacylglycerol
PA is formed from glycerol-3-phosphate (G3P), which is primarily formed by DHAP
the reaction is regulated with 1 un/saturated enzyme, so that 2 AA are connected with ester bonds.
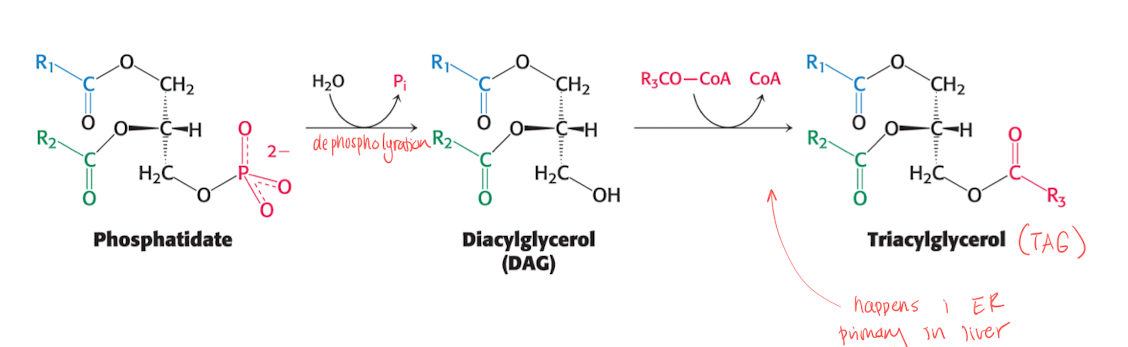
Phosphatide (PA) —> Triacylglycerol (TAG)
PA is de-phosphorylated to form TAG. A 3rd FA is activated by CoA, replaces the phosphate group in PA. The reaction happens primarily in the liver cells ER mmembrane.
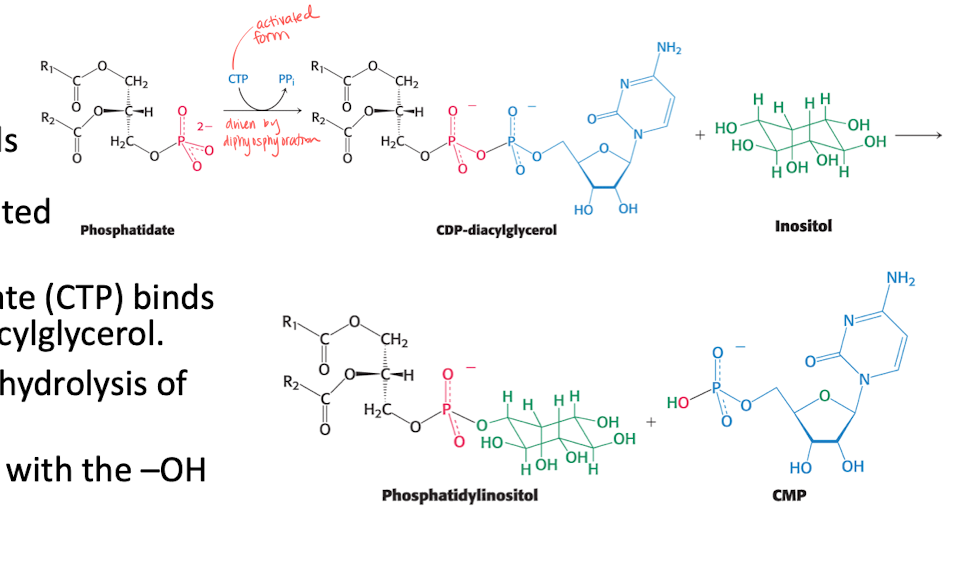
Phosphatide (PA) —> Phospholipids.
A activated intermediate is needed, CTP binds to PA to form CDP diacylglycerol (with hydrolysis).
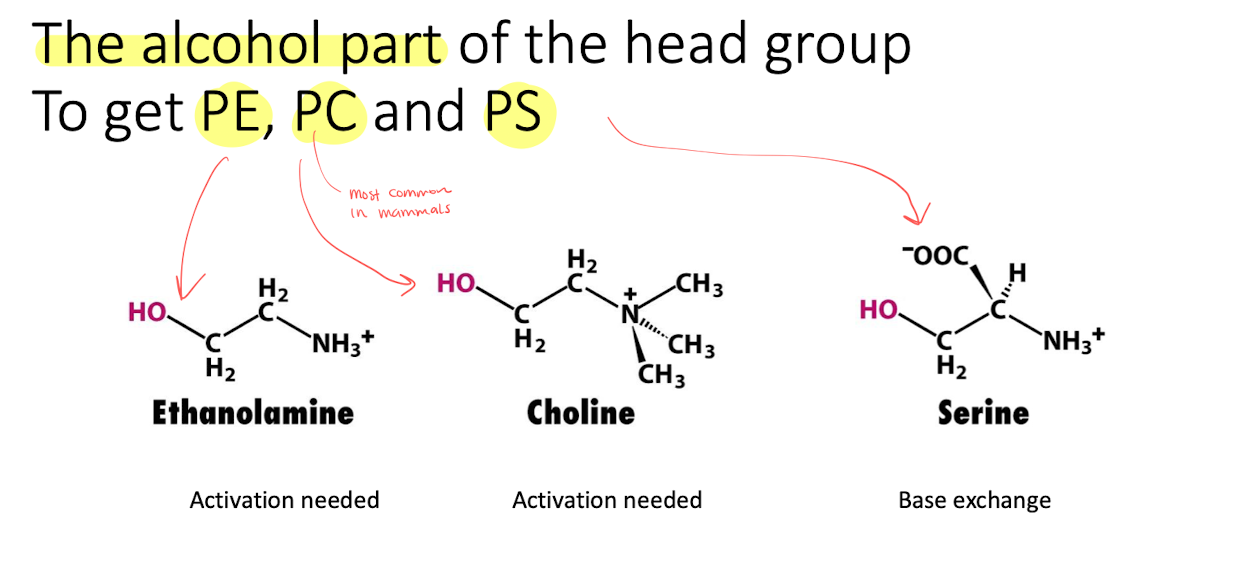
the activated PA then reacts with -OH of alcohol. (We can get PE, PC, PS) depending on the structure of the alcohol.
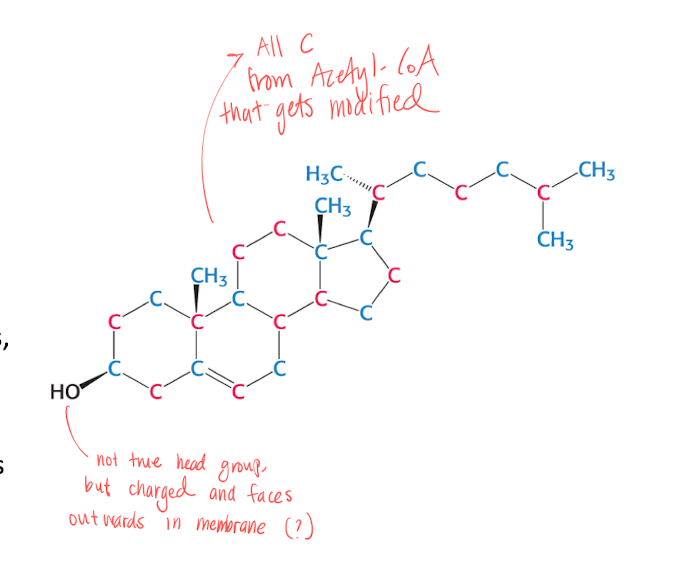
What is cholesterol?
C27H46O
Steroid molecule
Controls about 30% of cell fluidity and bidrar till produktionen av biel salts, steroid hormones and Vit D.
Both Exogenous and Endogenous
The -OH gives the molecule a charge, and is faced outwards in the membrane.
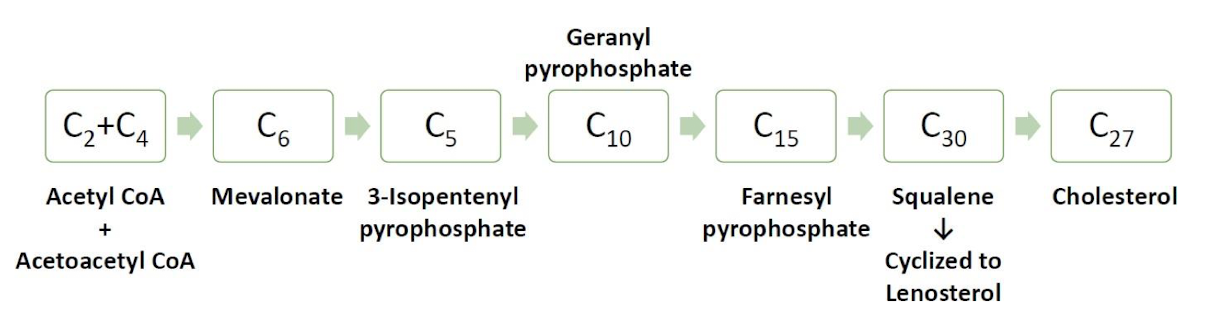
What are the 3 stages of cholesterol synthsis?
All 27C comes from Acetyl-CoA
Synthesis of isopentenyl pyrophosphate in cytoplasm
Condensation to squalene in ER
convertion to cholesterol in ER
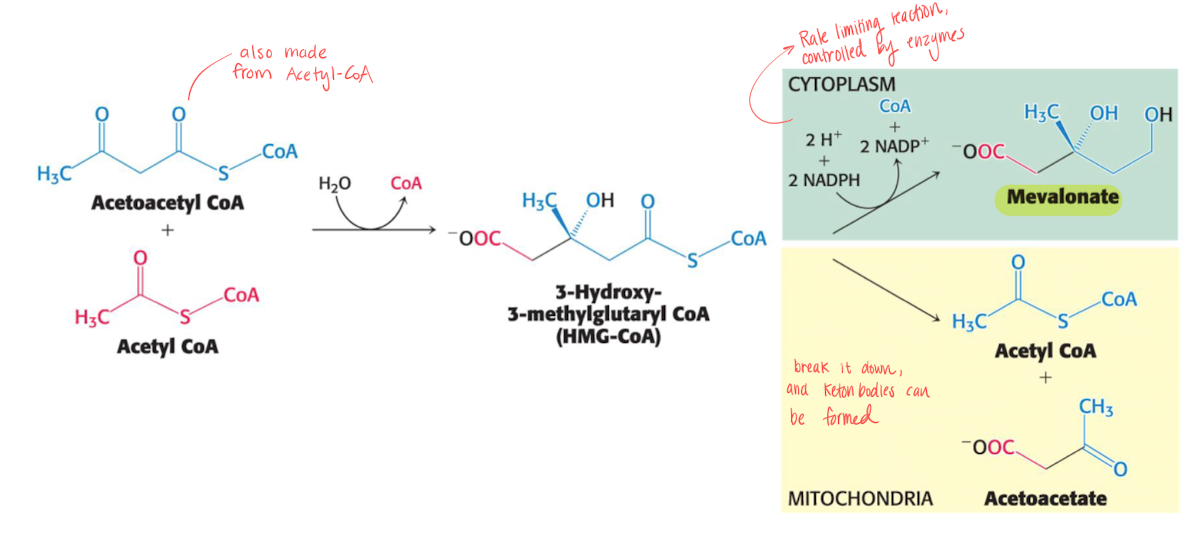
Stage 1 - 3-isopentyl pyrophosphate
Acetyl-CoA and Acetoacetyl-CoA (also from acetyl-CoA) forms mevalonate using 2 NADPH, this step is rate limiting and occurs in cytoplasm.
Mevalonate is then turned into 3-isopentyl pyrophosphate using 3 ATP.
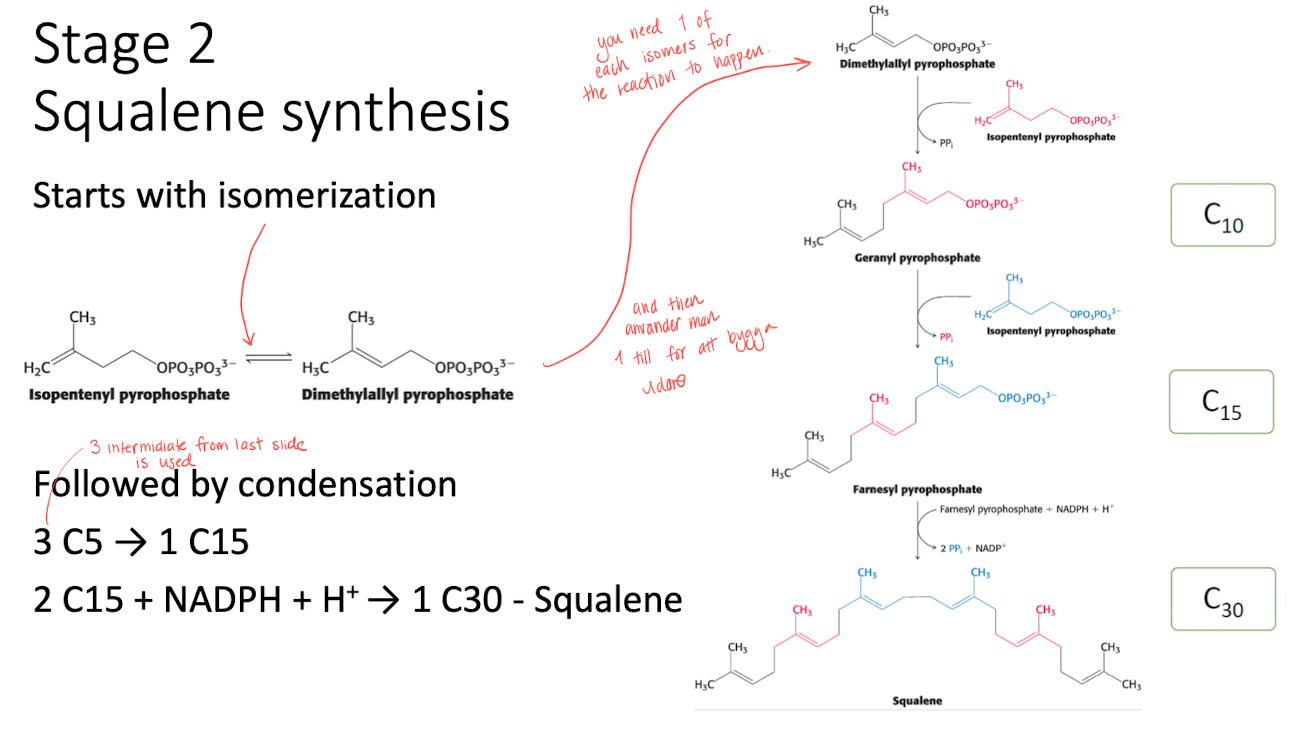
Stage 2 - squalene synthesis
Isomerization of isopentyl pyrophosphate into demetylallyl pyrophospahte. 1 of each is needed to start the reaction.
Followed by condensation to form squalene using 1 NADPH.
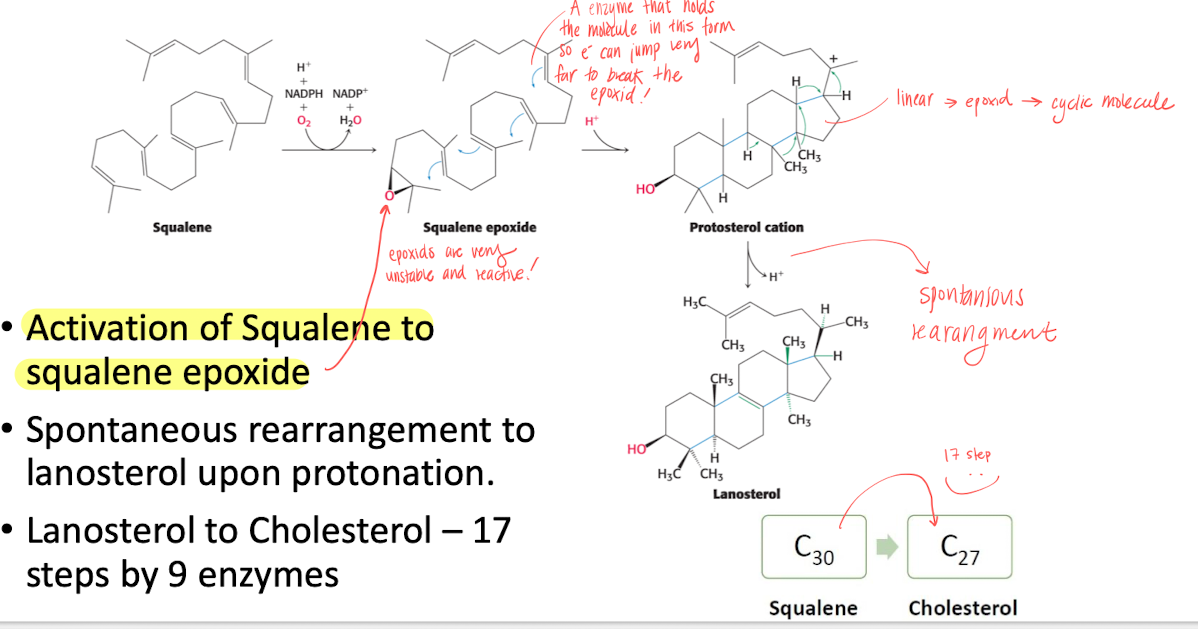
Stage 3 - Cyclization to form cholesterol
Activating of squalene by forming an epoxid with an enzyme that holds squalene in place for e- to jump.
Epoxid are super unstable, so reactions happens and finally spontaneous rearrangment to lanosterol upon protonation.
then turned into cholesterol in 17 steps.
Regulation of cholesterol biosynthsis - main focus on SREBP
It is a transcription factor that senses the level off presented cholesterol. it has a DNA-binding domain.
When [Cholesterol] is low, SREBP will move from ER to Golgi, and the DNA-binding domain wil be cleaved and will bind to SRE to enhance gene expression.
high [Cholesterol] —> cleavage blocked, won’t enter Golgi and no enhencment of expression.
![<p>It is a <strong>transcription factor </strong>that senses the level off presented cholesterol. it has a <mark data-color="#fbfcc9" style="background-color: #fbfcc9; color: inherit">DNA-binding domain</mark>. </p><ul><li><p>When [Cholesterol] is low, SREBP will move from ER to Golgi, and the DNA-binding domain wil be cleaved and will bind to SRE to enhance gene expression. </p></li><li><p>high [Cholesterol] —> cleavage blocked, won’t enter Golgi and no enhencment of expression. </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/1790c39c-acb0-4bc8-8dd9-3e96a40ac0f7.png)
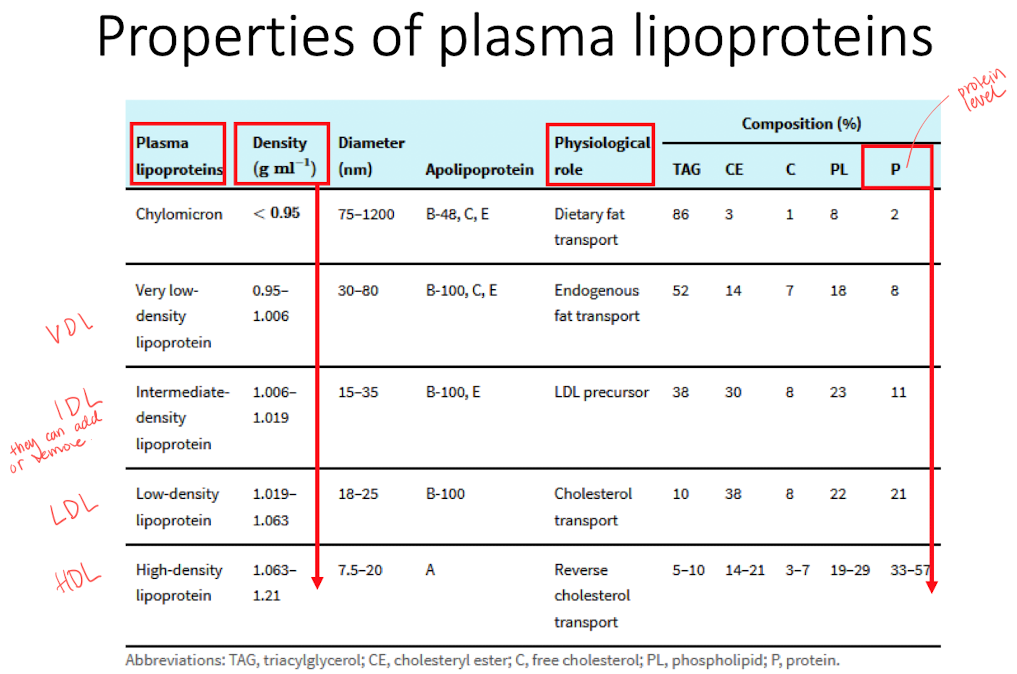
Lipoproteins
Core built by hydrophobic lipids that is surrouded by polar lipids and proteins.
Important for transport of hydrophobic molecules and cholesterol homeostasis (maintain stable conditions in the cells).
Classified according to density, different classes has different roles. The class can be shifted by picking / releasing cargos.
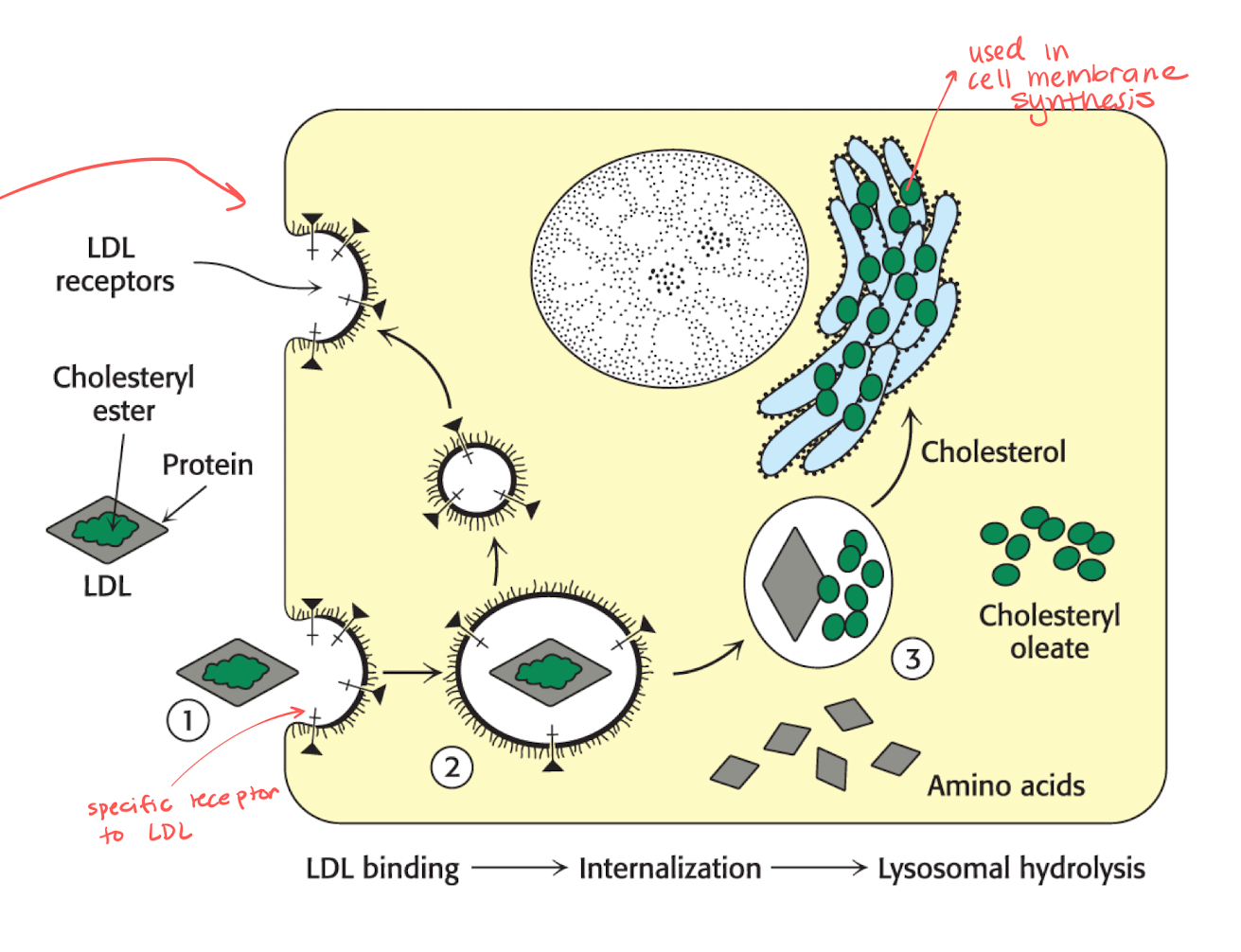
What is LDL’s role in cholesterol metabolism?
They work as the main cholesterol transporters.
There are specific LDL receptors that can be recycled, the numbers of these increases when the level of cholesterol is low.
Receptors will catch the LDL that carries cholesteryl ester in proteins.
The LDL is taken into the cell and the receptors are released and reused.
The protein inside of LDL is broken down into AA and the green part becomes cholesteryl oleate.
The green things turns into cholesterol and is used for membrane synthesis in the ER.
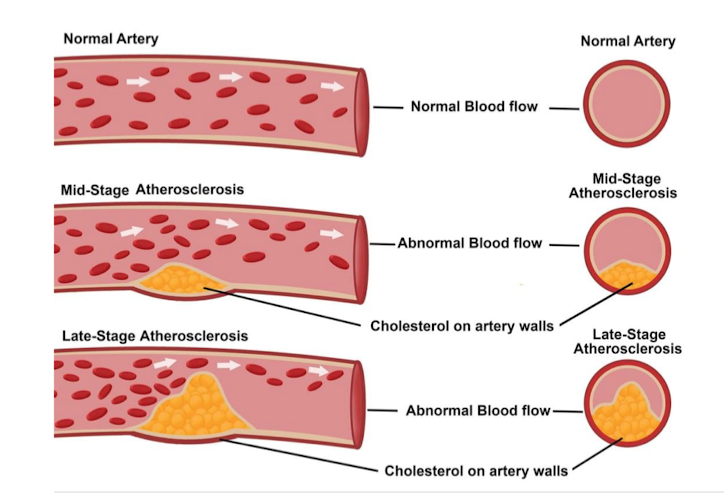
Atherosclerosis
blockage in the blod vessels due to increased plasma LDL, which leads to plaque, results in decreased blood flow.
treatment
block de nova synthesis of cholesterol. T.ex inhibitors that blocks the production of mevalonate in stage 1 of production of cholesterol.
better diet.
LDL (bad cholesterol) VS. HDL (good cholesterol)
LDL
high level associated with atherosclerosis and coronary heart disease.
may react with free radicals to form oxidized LDL, which triggers inflammation and increased risk of plaque formation.
transported from the liver to the cells
HDL
Reversed cholesterol transport, which removes the cholesterol to the liver.
It inhibits LDL-oxidation.
Cholesterol as precursor
bile salts
Steroid hormones : testosterone, cortisol, Estradiol etc.
Vitamin D
Caloric homeostasis
Ability to maintain adequate, but not excessive, energy stores.
consumed = expended + stored.
basal metabolic rate
Happening constaintly
The amount of energy needed to keep the body functioning at rest (50-70%)
Physical activity level
Wall, stand etc (20-40%)
diet included thermogenesis
thermic effect of food (5-15%)
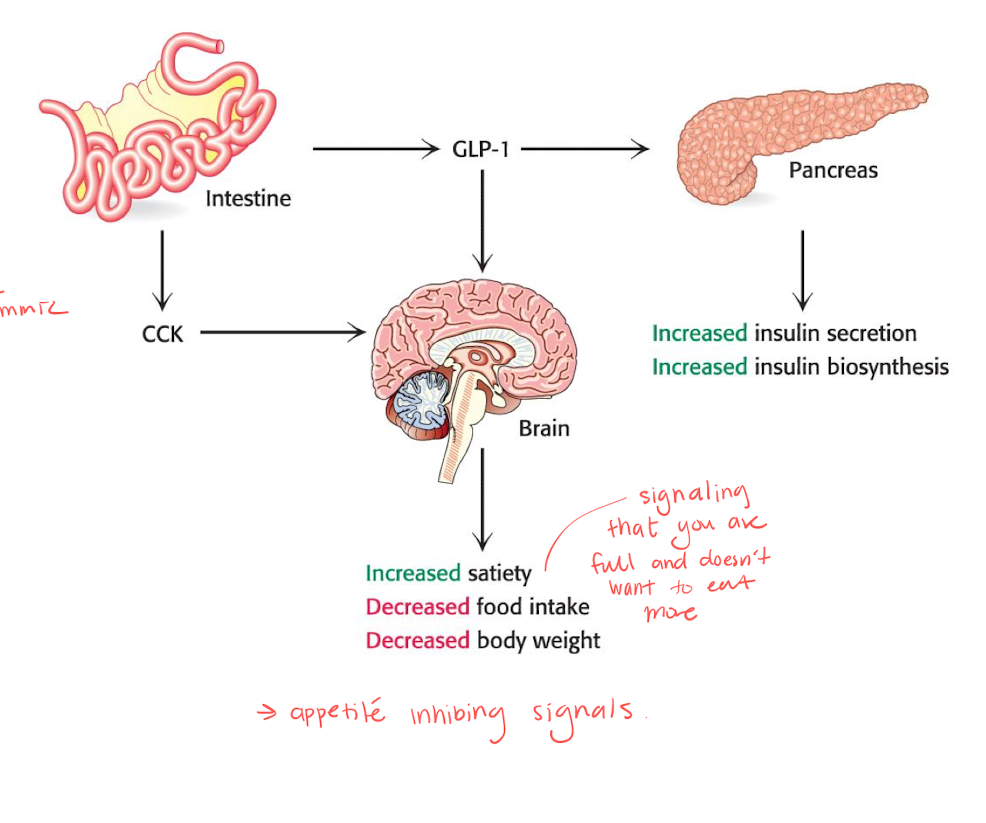
Short term control of caloric homeostasis - CCK and GLP-1
the famous ozempic tries to mimmic GLP-1.
these are small peptide hormones that signals satiety.
gives appetite inhibiting signals, increasing satiety, and decreases food intake and body weight.
CCK
induces secretion of pancreatic enzymes and bile salts.
GLP-1
enhances insulin secretion and inhibits glucagon secretion.
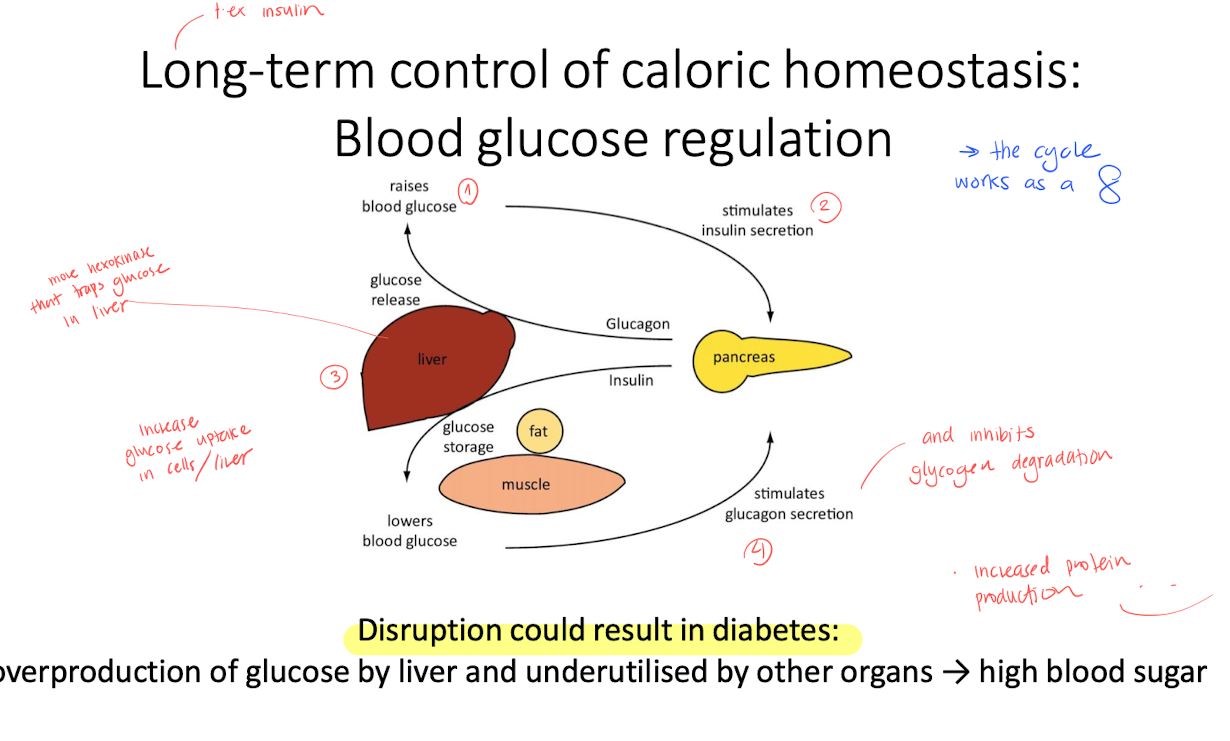
Long term control of caloric homeostasis - Leptin and insulin
the cycle goes around like a “8”, if the process is disruped, it might cause diabetes.
Leptin
control TAG storage
supresses appetite by affecting the brain
high leptin increases insulin sensitivity, decreses TAG synthesis.
Insulin
controls both blood glucose level and affects the appetite.
SOCS : blocking insulin signaling, and bnlocks degradation of receptors and IRS.
Diabetes
Type 1
no insulin production
Type 2
Produces insulin, but not accepted
Obesity is a main risk factor, since it limits how much lipids can be hold in the alipocide, this causes lipids to enter other tissues, increasing chance of type 2 diabetes.
treatment
- Metformin
- semaglutide
- exercise bitch
Excess FAs will modifies the metabolism
less glycogen synthesis
less glucose uptake
Less active insulin receptors —> decreased insulin signaling.
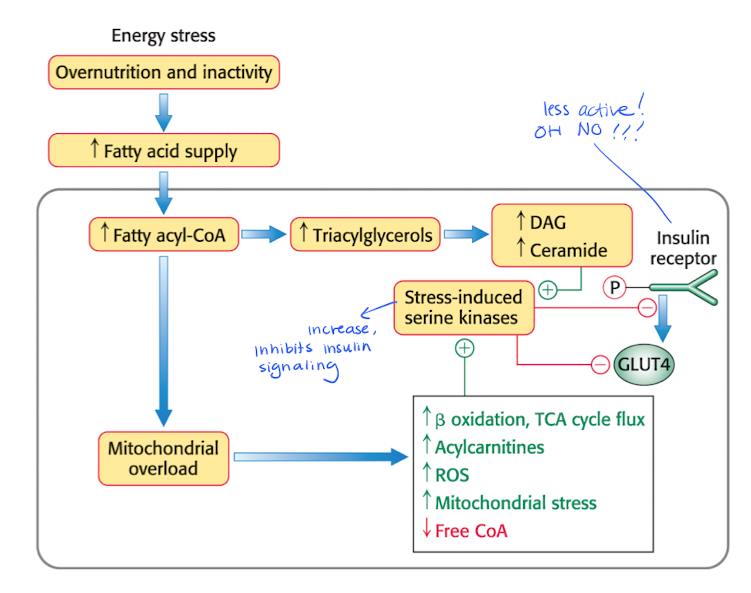
Insulin resistance and pancreatic failure
Insulin released by pancreas is regulated by the blood glucose conc (with ATP and ion channels)
High [glucose] e.g high lipid levels stimulates insulin synthesis and secretion ( high ATP = changes potential = insulin release)
The insulin are produced as pre-insulin, too much of those causes ER stress because the body can’t process that much insulin at the same time. ER tries to produce even more insulin.
Too much insulin —> increased missfolding of proteins —> incresed chaperones —> increased breakdown of wrong proteins.
Unfolded proteins —> UPR If ER stress can’t be fixed, them optosis (programmed cell death) will occur.
![<p>Insulin released by pancreas is regulated by the blood glucose conc (with ATP and ion channels)</p><ul><li><p>High [glucose] e.g high lipid levels stimulates insulin synthesis and secretion ( high ATP = changes potential = insulin release)</p></li><li><p>The insulin are produced as <strong>pre-insulin</strong>, too much of those causes <strong>ER stress</strong> because the body can’t process that much insulin at the same time. ER tries to produce even more insulin. </p></li><li><p>Too much insulin —> increased missfolding of proteins —> incresed chaperones —> increased breakdown of wrong proteins.</p></li><li><p>Unfolded proteins —> <strong>UPR </strong>If ER stress can’t be fixed, them <strong>optosis (programmed cell death) </strong>will occur. </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/3a9cf9a2-6c6d-4302-828b-7a997ab032d7.png)
What is protein wasting? - starvation
When you are missing some of the essential AAs, which means that even though you consume proteins, a lot goes into waste.
Often a consequence of starvation.
when the glucose source is used, the body tries to find other sources, which creates ketone bodies.
Once the lipid source is out in the adipocytes, proteins (heart, liver etc) will degrade —> death.