Biology 1 Chapter 7
1/95
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
96 Terms
It has been thought that many diseases now associated with aging are related to malfunctioning mitochondria. Why are the mitochondria so important to all cells?
a. They produce energy in the form of ATP.
b. They carry out anaerobic respiration.
c. They are the source of all human disease.
d. They are extremely large.
e. They are located only in vital organs.
a. They produce energy in the form of ATP.
When individuals have mitochondrial disorders, why are the skeletal and heart muscles and the brain most often
affected?
a. They are the most important organs.
b. They have the highest energy needs.
c. They are generally very fragile.
d. They have fewer mitochondria in the cell.
e. They are the most complex organs.
b. They have the highest energy needs.
When a molecule is reduced, it ____.
a. gains electrons
b. loses electrons
c. stores electrons
d. loses energy
e. burns energy
a. gains electrons
Where would you expect to find proteins responsible for controlling the substances that enter and leave mitochondria?
a. plasma membrane of the cell
b. inner mitochondrial membrane
c. outer mitochondrial membrane
d. nuclear membrane
e. lysosomal membrane
b. inner mitochondrial membrane
What is the ultimate fate of the atoms in oxygen gas (O2) in cellular respiration?
a. They are respired as CO2.
b. They are incorporated to water.
c. They are attached to glucose.
d. They are attached to pyruvate.
e. They accepts electrons in glycolysis.
b. They are incorporated to water.
Alzheimer disease has been shown to be related to ____.
a. alcoholic fermentation in the brain
b. lactate fermentation in the brain
c. increased brain metabolism
d. decreased brain metabolism
e. increased mitochondrial enzyme activity
d. decreased brain metabolism
In the process of aerobic metabolism, carbon containing molecules are broken down and the energy from the electrons
is used to ____.
a. directly supply the energy needs of an organism
b. alter enzyme structure
c. generate a proton gradient
d. heat the organism in a cold environment
e. supply heat to stored fat
c. generate a proton gradient
Which answer best describes energy flow in biological systems?
a. glucose --> G3P --> NADH --> ATP
b. bacteria --> archaea --> plants --> animals
c. NAD+
--> NADH --> ADP --> ATP
d. G3P --> glucose --> ATP --> NAD+
e. pyruvate oxidation --> glycolysis --> fermentation --> citric acid cycle
a. glucose --> G3P --> NADH --> ATP
How does cellular respiration differ in prokaryotes and eukaryotes?
a. Eukaryotes use substrate-level phosphorylation; prokaryotes use oxidative phosphorylation.
b. Eukaryotes perform reactions in mitochondria; prokaryotes use the plasma membrane.
c. Eukaryotes use NAD+
/NADH as electron acceptors; prokaryotes use FAD+
/FADH2.
d. Eukaryotes do not use oxygen; prokaryotes only use oxygen.
e. Eukaryotes metabolize only glucose; prokaryotes metabolize only galactose.
b. Eukaryotes perform reactions in mitochondria; prokaryotes use the plasma membrane.
What type of chemical reaction must occur for electrons to flow from one molecule to the next and supply the energy
for metabolism?
a. acid/base
b. reduction/oxidation
c. exothermic
d. trimolecular
e. phosphorylation
b. reduction/oxidation
. If the inner membrane of the mitochondria were compromised in some way, what effect would this have on cellular
respiration?
a. The transport of electrons across the inner mitochondrial membrane would not occur.
b. The proton gradient across the inner mitochondrial membrane would dissipate.
c. The ATP synthase enzyme would relocate to the mitochondrial matrix.
d. The cell would generate more ATP.
e. ATP would no longer be made anywhere in the cell by any mechanism.
b. The proton gradient across the inner mitochondrial membrane would dissipate.
At the end of cellular respiration, oxygen combine with electrons of a very low energy level. How are the specific
properties of oxygen beneficial to the organism that uses it as a final electron acceptor?
a. Oxygen is highly reactive and readily accepts electrons.
b. Oxygen is strongly electronegative and helps pull the electrons through the electron transport chain.
c. Oxygen allows a maximum output of energy for ATP synthesis.
d. Oxygen is the only molecule that can act as a final electron acceptor.
e. Oxygen is highly reactive and readily oxidizes methane.
c. Oxygen allows a maximum output of energy for ATP synthesis.
Oxidative phosphorylation is the process by which ____.
a. high energy NADH is made to supply the cell with its needed energy
b. a final electron acceptor is used indirectly to facilitate the production of ATP
c. ATP is made using high energy intermediates of cellular respiration
d. specific enzymes are regulated to control cellular respiration
e. NAD+
is regenerated to allow glycolysis to continue
b. a final electron acceptor is used indirectly to facilitate the production of ATP
In the absence of ATP synthase, animal cells would not be able to ____.
a. create a proton gradient
b. hydrolyze glucose to G3P
c. carry out oxidative phosphorylation
d. produce ATP
e. carry out pyruvate oxidation
d. produce ATP
How are NADH and FADH2 similar?
a. They both directly produce ATP.
b. They are both used in glycolysis.c. They both contain high energy phosphates.
d. They both contain high energy electrons.
e. They are both in the oxidized form.
d. They both contain high energy electrons.
What supplies the electrons for oxidative phosphorylation?
a. ATP
b. NADH and FADH2
c. glucose
d. the proton gradient
e. ATP synthase
b. NADH and FADH2
During glycolysis, glucose molecules are broken down by breaking the carbon-hydrogen bonds that are present and
forming carbon-oxygen bonds. In this process, glucose is ____.
a. partially oxidized
b. partially reduced
c. completely oxidized
d. completely reduced
e. hydrolyzed
a. partially oxidized
The initial step of glycolysis involves the ____ of glucose.
a. condensation
b. hydrolysis
c. oxidation
d. phosphorylation
e. reduction
d. phosphorylation
. The enzymes responsible for hydrolyzing glucose into glyceraldehyde-3-phosphate (G3P) are found in which part of
the cell?
a. cytosol
b. mitochondria
c. rough ER
d. nucleus
e. cell membrane
a. cytosol
The final product of glycolysis is ____.
a. glucose
b. fructose
c. glyceraldehyde-3-phosphate
d. pyruvate
e. carbon dioxide
d. pyruvate
A toxic substance has been found to inhibit glucose transport into mammalian cells. If this substance is administered
to a hamster, the likely cause of death would be a lack of ____ production.
a. NAD+
b. ATP
c. GTP
d. CO2
e. FAD
b. ATP
Which of these molecules has the most potential energy?
a. glucose
b. pyruvate
c. ATP
d. NADH
e. FADH2
a. glucose
Ultimately, the carbon molecules in pyruvate end up in which molecule?
a. NADH
b. acetate
c. ATP
d. CoA
e. CO2
e. CO2
What is the function of NADH and FADH2?
a. Both release energy for glycolysis to proceed forward.
b. Both provide electrons to the electron transfer system.
c. Both produce ATP by substrate-level phosphorylation.
d. NADH delivers electrons, while FADH2 supplies H+
e. NADH is found only in the cytosol and FADH2 only in the matrix.
b. Both provide electrons to the electron transfer system.
During which stages of cellular respiration is CO2 released?
a. glycolysis
b. pyruvate oxidation
c. citric acid cycle
d. electron transport system
e. both pyruvate oxidation and citric acid cycle
e. both pyruvate oxidation and citric acid cycle
Patients with pyruvate dehydrogenase complex deficiency develop neurological symptoms. Patients with this disease
fail to make the substrates required for which metabolic process?
a. pyruvate oxidation
b. oxidative phosphorylation
c. glycolysis
d. the citric acid cycle
e. fatty acid oxidation
d. the citric acid cycle
Which molecule is responsible for carrying the acetyl group from pyruvate into the citric acid cycle?
a. NADH
b. FADH2
c. ATP
d. CoA
e. oxaloacetate
d. CoA
Per molecule of glucose, oxidation occurs ____ times during the conversion of pyruvate to acetyl CoA.
a. 1
b. 2
c. 3
d. 4
e. 5
b. 2
The final step of the citric acid cycle oxidizes malate to oxaloacetate and reduces NAD+
. What is the purpose of this
step?
a. to replenish the supplies of NAD+
b. to replenish free CoA
c. to regenerate oxaloacetate to attach another acetate molecule
d. to produce ATP by substrate-level phosphorylation
e. to produce ATP by oxidative phosphorylation
c. to regenerate oxaloacetate to attach another acetate molecule
What is the fate of CoA after it delivers an acetyl group into the citric acid cycle?
a. It is degraded and used for energy.
b. It is recharged with another acetate.
c. It is used in protein synthesis.
d. It remains in an inactive form until the cell dies.
e. It is reused to start glycolysis.
b. It is recharged with another acetate.
Citrate synthase, the first enzyme in the citric acid cycle, is regulated by ATP concentrations. Why is early regulation
of ATP production the most beneficial method for the cell?
a. It is not possible to regulate the last enzyme in a pathway.
b. More ATP can be produced before the first enzyme is inactivated.
c. ATP production always needs to be maximized.
d. Citrate synthase controls the amount of pyruvate produced by glycolysis.
e. Regulating early steps conserves cellular fuels.
e. Regulating early steps conserves cellular fuels.
For every glucose molecule that goes through cellular respiration, how many carbon atoms are fully oxidized to CO2
in the citric acid cycle?
a. 1
b. 2
c. 3
d. 4
e. 5
d. 4
Which molecule(s) is/are responsible for delivering the high energy electrons from the citric acid cycle to the electron transfer system?
a. NADH only
b. FADH2 only
c. Both NADH and FADH2
d. Cyt C and Q
e. ATP and ADP
c. Both NADH and FADH2
Which molecule is the final electron acceptor in respiration?
a. water
b. ATP
c. carbon dioxide
d. oxygen
e. hydrogen
d. oxygen
Glycolysis, pyruvate oxidation, and the citric acid cycle all produce ____.
a. CO2
b. H2O
c. ATP
d. FADH2
e. NADH
e. NADH
Compared to the mitochondrial intermembrane space, the mitochondrial matrix could be described as having ____ pH
and _____ pyruvate concentration.
a. a lower; higher
b. a higher; higher
c. a lower; lower
d. a higher; lower
e. the same; the same
d. a higher; lower
What are the functions of cytochrome c and ubiquinone?
a. They translocate protons from the matrix to the inner mitochondrial space.
b. They shuttle electrons between the protein complexes.
c. They synthesize water from molecular oxygen.
d. They produce ATP by substrate-level phosphorylation.
e. They produce ATP by oxidative phosphorylation.
b. They shuttle electrons between the protein complexes.
Free radicals, highly reactive species with unpaired electrons that damage molecules and cells, and can contribute to
aging. Coenzyme Q (also known as ubiquinone or CoQ) is often found in lotions and moisturizers. Given what you know
about the role of CoQ in cellular respiration, why would it be added to these products?
a. It recruits free radicals to help increase the rate of glycolysis in skin cells.
b. It absorbs free radicals that can cause damage to skin cells.
c. Removing free radicals decreases the rate of metabolism and slows growth of skin cells.
d. It allows for the regeneration of new skin cells.
e. It drives the spontaneous death of older skin cells that have accumulated too many free radicals.
b. It absorbs free radicals that can cause damage to skin cells.
The enzyme succinate dehydrogenase, located in the inner mitochondrial membrane, is directly involved in ____.
a. glycolysis and pyruvate oxidation
b. pyruvate oxidation and citric acid cycle
c. citric acid cycle and electron transfer system
d. electron transfer system and glycolysis
e. electron transfer system and fermentation
c. citric acid cycle and electron transfer system
What is directly responsible for pumping protons out of the mitochondrial matrix?
a. protein complexes I, II, III, and IV
b. cytochrome c and ubiquinone
c. protein complexes I and III
d. protein complexes I, III, and IV
e. NADH and FADH2
d. protein complexes I, III, and IV
What is the proton-motive force?
a. the force needed to move protons into the inner mitochondrial space
b. the amount of energy required to protonate a glucose molecule
c. the free energy associated with the removal of hydrogen from NADH
d. the combination of a proton and voltage gradient across the inner mitochondrial membrane
e. the synthesis of ATP from a proton gradient
d. the combination of a proton and voltage gradient across the inner mitochondrial membrane
What powers ATP synthase directly?
a. electron transfer
b. NADH and FADH2
c. carbohydrate metabolism
d. the proton gradient
e. protein complexes
d. the proton gradient
Where is ATP synthase located in non-photosynthetic eukaryotes?
a. outer membrane of the cell
b. nuclear envelope
c. rough endoplasmic reticulum
d. matrix of the mitochondria
e. inner mitochondrial membrane
e. inner mitochondrial membrane
What directly supplies the electrons for the electron transfer system?
a. ATP and ADP
b. FADH2 and NADH
c. pyruvate and acetate
d. various enzymes
e. oxygen and water
b. FADH2 and NADH
To reach the mitochondrial matrix, protons travel through a channel made by the ____ of ATP synthase.
a. basal unit
b. headpiece
c. stalk
d. lollipop
e. three catalytic sites
a. basal unit
When the H+ concentration is significantly higher in the mitochondrial matrix than the intermembrane space, ATP synthase would ____.
a. require an ion to stabilize it
b. no longer function properly
c. hydrolyze ATP to form ADP to pump protons out of the matrix
d. generate ATP to pump protons into the matrix
e. be uncoupled from the electron transport chain
c. hydrolyze ATP to form ADP to pump protons out of the matrix
Which part of the ATP synthase is responsible for catalyzing ATP formation?
a. the basal unit
b. the headpiece
c. the stalk
d. the lollipop
e. the electrons
b. the headpiece
A typical eukaryotic cell that has an abundant supply of glucose and O2 will generate a proton gradient in its
mitochondria by ____ that is used primarily for ____.
a. chemiosmosis; substrate-level phosphorylation
b. the electron transport chain; chemiosmosis
c. the electron transport chain; substrate-level phosphorylation
d. fermentation; NAD reduction
e. glycolysis; production of CO2
b. the electron transport chain; chemiosmosis
What is one potential fate for a proton in the mitochondrial matrix?
a. They are attached to NAD+
and FAD.
b. They combine with oxygen to form water.
c. They synthesize ATP by substrate-level phosphorylation.
d. They help in the production of CO2.
e. They regenerate Coenzyme A.
b. They combine with oxygen to form water.
Why does NADH produce more energy than FADH2?
a. FADH2 donates electrons to protein complex III as opposed to complex II.
b. FADH2 requires more ATP to produce it and gives more energy back.
c. NADH and FADH2 are synthesized in different steps of cellular respiration.
d. NADH has a high free energy and can be oxidized more readily than FADH2.
e. NADH supplies fewer electrons that are of a higher energy state than FADH2.
d. NADH has a high free energy and can be oxidized more readily than FADH2.
In order to completely oxidize glucose, it takes two turns of the citric acid cycle, which yields a net of 2 ATP, 6
NADH and 2 FADH2. How many of the 32 total ATP molecules produced in cellular respiration come from the citric acid
cycle, including the contribution from the NADH and FADH2?
a. 32
b. 28
c. 24
d. 20
e. 16
d. 20
How efficient is cellular respiration in extracting the energy stored in the bonds of glucose?
a. 25%
b. 33%
c. 45%
d. 50%
e. 80%
b. 33%
Suppose a human metabolic disease only allows electrons to be used from NADH and not FADH2. What is a
probable cause of this disease?
a. a defect in assembly protein genes for complex II of the electron transfer system
b. enzyme defects in glycolysis and the citric acid cycle
c. a deficient amount of cytochrome c and coenzyme Q
d. improper regulation of phosphofructokinase
e. inability of oxygen to act as a final electron acceptor
a. a defect in assembly protein genes for complex II of the electron transfer system
Suppose we hypothesize that potato plants use uncoupling proteins (UCPs) in a similar way as mammals. What would be the evidence to support this hypothesis?
a. increased amounts of ATP production
b. decreased sugar metabolism
c. increased internal tissue temperature
d. decreased mitochondrial catabolism
e. increased cytosolic pH
c. increased internal tissue temperature
Racker and Stoeckenius used synthetic phospholipid membrane vesicles that contained a light-activated protein pump
and ATP synthase to test Mitchell's chemiosmotic hypothesis. What was the conclusion of this experiment?
a. Phospholipid membrane vesicles require a proton gradient to maintain integrity.
b. ATP synthase is powered by the proton-motive force.
c. ATP synthase can generate a proton gradient.
d. Light-activated proton pumps can generate a proton gradient.
e. Light-activated proton pumps interact with ATP synthase to modulate its activity
b. ATP synthase is powered by the proton-motive force.
A patient with a mitochondrial disease is found to have a mutation in Gene X. A homologous gene in a Drosophila
(fruit fly), did not cause a similar deficit. What does this suggest?
a. Gene X is highly conserved between humans and Drosophila.
b. Gene X is not conserved between humans and Drosophila.
c. Gene X is encoded in nuclear DNA.
d. Gene X is encoded in mitochondrial DNA.
e. Gene X is a redundant protein in Drosophila.
b. Gene X is not conserved between humans and Drosophila.
As a result of fermentation, cells produce ____.
a. ADP
b. NADH
c. FAD
d. O2
e. NAD+
e. NAD+
In the absence of O2, the partial metabolism of glucose in human muscles produces _____.
a. acetaldehyde
b. carbon dioxide
c. energy
d. lactic acid
e. oxygen
d. lactic acid
. After one minute without oxygen, brain cells begin to die. After three minutes, this damage is likely to cause severe
neurological deficits. The dependence of brain cells on oxygen for survival indicates that these cells are ____.
a. strict aerobes
b. strict anaerobes
c. facultative aerobes
d. facultative anaerobes
e. transitional aerobes
a. strict aerobes
Anaerobic respiration produces ATP by _____.
a. glycolysis only
b. glycolysis and the Krebs only
c. glycolysis, the Krebs and electron transport chain with inorganic molecules as a final acceptor
d. glycolysis, the Krebs and electron transport chain with organic molecules as a final acceptor
e. electron transport chain with organic molecules as a final acceptor only
c. glycolysis, the Krebs and electron transport chain with inorganic molecules as a final acceptor
The Warburg effect is the observation that cancer cells produce energy using a high rate of glycolysis followed by fermentation rather than oxidative phosphorylation. Although the exact cause is still under investigation, which explanation is most plausible?
a. Tumors attract blood vessels to increase levels of available oxygen.
b. In order to grow quickly, high levels of ATP are required.
c. Mutations in phosphofructokinase prevent feedback inhibition.
d. Tumor formation upregulates glycolytic proteins.
e. Rapid cell proliferation damages mitochondrial function.
e. Rapid cell proliferation damages mitochondrial function.
About 10-20% of patients with Leigh Syndrome, a mitochondrial disease, have a mutation in MT-ATP6, a gene that
codes for ATP synthase. These patients often experience high levels of _____ in their cells due to an increase in levels of
pyruvate that are unable to convert to acetyl-CoA.
a. NADH
b. FADH2
c. lactate
d. ethanol
e. carbon dioxide
c. lactate
Some organisms are not able to live in an environment where there is oxygen; these types of organisms are called
obligate anaerobes. Which explanation is most plausible for how they survive without oxygen?
a. They are able to survive using less energy than aerobes.
b. All of their ATP is imported into the cell from an external source.
c. Sulfur is used instead of oxygen because it is chemically similar.
d. These organisms use photosynthesis to produce energy.
e. Their mitochondria are damaged, and consequently they are short-lived.
c. Sulfur is used instead of oxygen because it is chemically similar.
Which enzyme in the glycolytic pathway acts as a switch that can be regulated by ATP, AMP, and citrate?
a. pyruvate kinase
b. triosephosphate isomerase
c. aldolase
d. ATP synthase
e. phosphofructokinase
a. pyruvate kinase
We study cellular respiration because it is one of the most important pathways in biology. In fact, nearly all
carbohydrates at some point in their catabolism are directed through cellular respiration. Why is it unnecessary to have
multiple independent pathways to break down different molecules?
a. Using cellular respiration is theoretically the most efficient way to break down sugars and other molecules.
b. Oxygen must be used in the breakdown of all molecules in order to yield ATP.
c. Greater complexity would lead to an eventual failure of the biological system.
d. Most biological cells only catabolize one or two different types of sugars and only need one main pathway.
e. Energy-containing macromolecules can be converted to products that can enter at various points in the cellular respiration pathway.
e. Energy-containing macromolecules can be converted to products that can enter at various points in the cellular respiration pathway.
The oxidation of which macromolecule yields the most energy by weight?
a. lipids
b. glycogen
c. starch
d. glucose
e. protein
a. lipids
Glucose biosynthesis is called _____.
a. glyconeogenesis
b. fatty acid oxidation
c. glycolysis
d. pentose phosphate pathway
e. gluconeogenesis
e. gluconeogenesis
When ATP levels are high, which enzyme's activity will be directly decreased by feedback inhibition?
a. aconitase
b. malate dehydrogenase
c. citrate synthase
d. isocitrate dehyrogenase
e. fumerase
c. citrate synthase
Which molecule stimulates phosphofructokinase to increase the flow of intermediates through glycolysis?
a. NADH
b. FADH2
c. ATP
d. AMP
e. acetyl CoA
d. AMP
When triglycerides are hydrolyzed, they are broken down into glycerol and fatty acids. The fatty acids are further
broken down into two-carbon fragments in a process called fatty acid oxidation. At which pathway do these fragments
enter respiration?
a. glycolysis
b. pyruvate oxidation
c. citric acid cycle
d. oxidative phosphorylation
e. carbohydrate hydrolysis
c. citric acid cycle
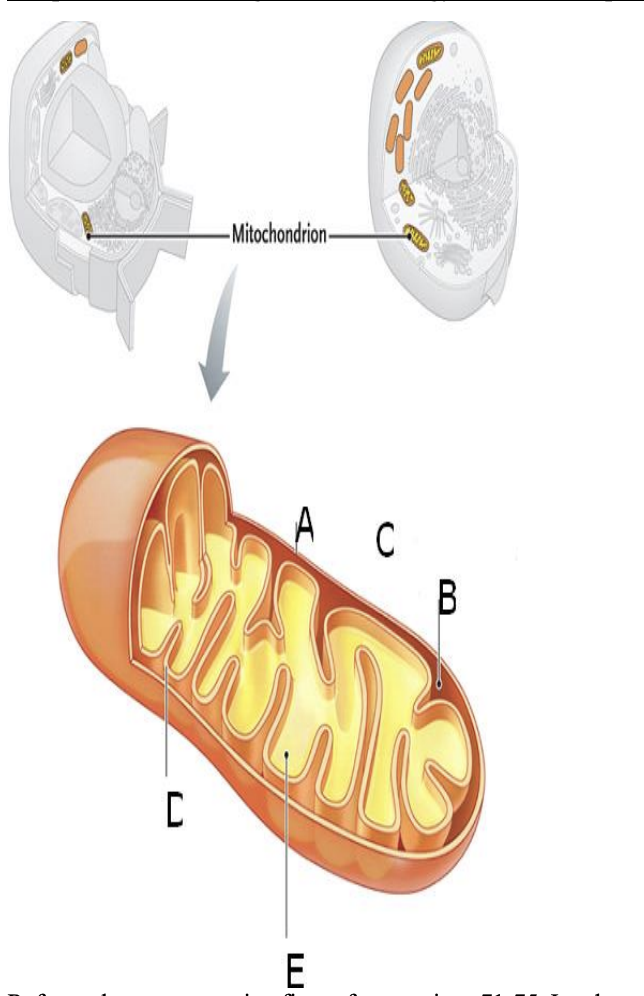
Refer to the accompanying figure for questions 71-75. In what part of the mitochondrion or cell do glycolysis, pyruvate oxidation, citric acid cycle, electron transfer system, ATP synthesis occur. Not all choices will be used; some may be used more than once.
a. outer mitochondrial membrane
b. intermembrane compartment
c. cytosol
d. inner mitochondrial membrane
e. matrix
c. glycolysis
e. pyruvate oxidation
e: citric acid cycle
d. electron transfer system
d. ATP synthesis
Match the following processes with the correct products.
a. glycolysis
b. the citric acid cycle
c. pyruvate oxidation
d. oxidative phosphorylation
e. fermentation
CO2, NADH, FADH2, ATP, H + , and CoA
b. the citric acid cycle
Match the following processes with the correct products.
a. glycolysis
b. the citric acid cycle
c. pyruvate oxidation
d. oxidative phosphorylation
e. fermentation
acetyl-coA, CO2 , NADH, and H +
c. pyruvate oxidation
Match the following processes with the correct products.
a. glycolysis
b. the citric acid cycle
c. pyruvate oxidation
d. oxidative phosphorylation
e. fermentation
8. NAD+ , FAD, and ATP
d. oxidative phosphorylation
Match the following processes with the correct products.
a. glycolysis
b. the citric acid cycle
c. pyruvate oxidation
d. oxidative phosphorylation
e. fermentation
pyruvate, ATP, NADH , H + , and H2O
a. glycolysis
Match the following processes with the correct products.
a. glycolysis
b. the citric acid cycle
c. pyruvate oxidation
d. oxidative phosphorylation
e. fermentation
lactate and NAD+
e. fermentation
Match the following coenzymes to their function.
a. NAD+
b. NADH
c. FADH2
d. CoQ
e. CoA
serves as an acetyl group carrier
e. CoA
Match the following coenzymes to their function.
a. NAD+
b. NADH
c. FADH2
d. CoQ
e. CoA
serves as an electron carrier and is oxidized in Complex I for oxidative phosphorylation
b. NADH
Match the following coenzymes to their function.
a. NAD+
b. NADH
c. FADH2
d. CoQ
e. CoA
serves as an electron carrier between complexes
d. CoQ
Match the following coenzymes to their function.
a. NAD+
b. NADH
c. FADH2
d. CoQ
e. CoA
serves as an electron carrier and is oxidized in Complex II for oxidative phosphorylation
c. FADH2
Match the following coenzymes to their function.
a. NAD+
b. NADH
c. FADH2
d. CoQ
e. CoA
serves as an electron carrier and is reduced throughout respiration
a. NAD+
In eukaryotes, where do the reactions of cellular respiration occur?
Glycolysis occurs in the cytosol. The other reactions occur in the mitochondria. Pyruvate oxidation and the citric acid cycle take place in the mitochondrial matrix, while the electron transport occurs in the inner mitochondrial membrane.
Both glycolysis and the citric acid cycle can be regulated by feedback inhibition. Provide two examples of feedback inhibition
In glycolysis, if excess ATP is present in the cytosol it binds to phosphofructokinase inhibiting fructose-6-phosphate from being converted to fructose-1, 6-bisphosphate. In the citric acid cycle the enzyme citrate synthase is inhibited by elevated ATP concentrations.
Explain why FADH2 generates less ATP than NADH.
NADH enters the mitochondrial electron transfer system at Complex I, while FADH2 enters at Complex II. Complex I pumps H+ into the intermembrane compartment whereas Complex II does not. Therefore, NADH can contribute additional H+ to the gradient, which will translate into an increase in proton motive force and increased ATP. Also, NADH contains an abundance of free energy (higher energy than FADH2) and can be oxidized readily.
How do cyanide and carbon monoxide affect cellular respiration?
Cyanide blocks the transfer of electrons from complex IV to oxygen. Carbon monoxide leads to abnormalities in mitochondrial function that inhibit complex IV activity.
What is the difference between a strict anaerobe and a facultative anaerobe?
Strict anaerobes cannot utilize oxygen as a final acceptor and generally live in oxygen free environments; therefore, they carry out fermentation. Facultative anaerobes can switch between fermentation and full oxidative pathways depending on oxygen supply.
If during a chemical reaction, a molecule loses hydrogens, it could be described as being oxidized.
a. True
b. False
true
Both plants and animals possess mitochondria, which are used for some cellular respiration pathways to make ATP.
a. True
b. False
true
Glycolysis takes place in the mitochondria.
a. True
b. False
false
Glycolysis yields a net of 2 pyruvate, 2 NADH, and 2 ATP.
a. True
b. False
true
During the process of pyruvate oxidation, pyruvate is broken down and the remaining two carbons are attached to
coenzyme A.
a. True
b. False
true
During glycolysis, ATP is produced by oxidative phosphorylation.
a. True
b. False
false
Two mobile electron carriers that are important for the electron transfer system are cytochrome c and coenzyme Q.
a. True
b. False
true
Products of fermentation include NADH and FADH2.
a. True
b. False
b. False
The key enzyme that regulates glycolysis by responding to changing levels of ATP is hexokinase.
a. True
b. False
false
The process of glucose biosynthesis is called gluconeogenesis.
a. True
b. False
true