Molecular & Cell Biology Exam 1
1/41
Earn XP
Description and Tags
BCOR 2500 Lectures 1-7
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
42 Terms
What is cellular reprogramming and why is it important
Can now reprogram adult somatic cells to become neurons in hopes of one day being able to replace parts of the brain like we replace parts of our cars.
E. Coli
Bacteria, easy to keep with a short life cycle and a simpler genome. Gave us an understanding for how DNA replication, gene expression and protein synthesis work.
Yeasts
Simplest eukaryotes,
Shared characteristics with E.coli: easy to keep, short life cycle, larger genome than e. coli but simpler than humans.
Shared characteristics with humans: distinct nucleus, genomic DNA organized in 16 linear chromosomes, contains organelles
C. elegans
Nematode (eukaryotic and multicellular), small number of genes and cells that have been mapped out, good to study animal development and cell differentiation, can use mutations to study developmental abnormalities (similar genes have been found in humans)
Drosophila melanogaster
Fruit fly, genome larger than c. elegans, easy to maintain, short reproductive cycle. Good to study the molecular mechanisms of development. Similar genes and mechanisms exist in humans
Arabidopsis thaliana
Simple plant, small genome, easy to grow and maintain. Methods for molecular genetic manipulations are available, identification of genes involved in plant development.
Zebrafish
Vertebrate, easy to maintain, reproduce rapidly, embryos develop outside the mother and are transparent. Several molecular techniques available to map mutations.
Mouse
mammal, more complex than other models, many mutations identified, several mutant mice available. More applicable to medicine: similar genomes, mutations in homologous genes result in similar phenotypes.
Cell culture
allows for controlled manipulations, makes it easier to study signaling mechanisms. Primary cultures vs immortal cell lines.
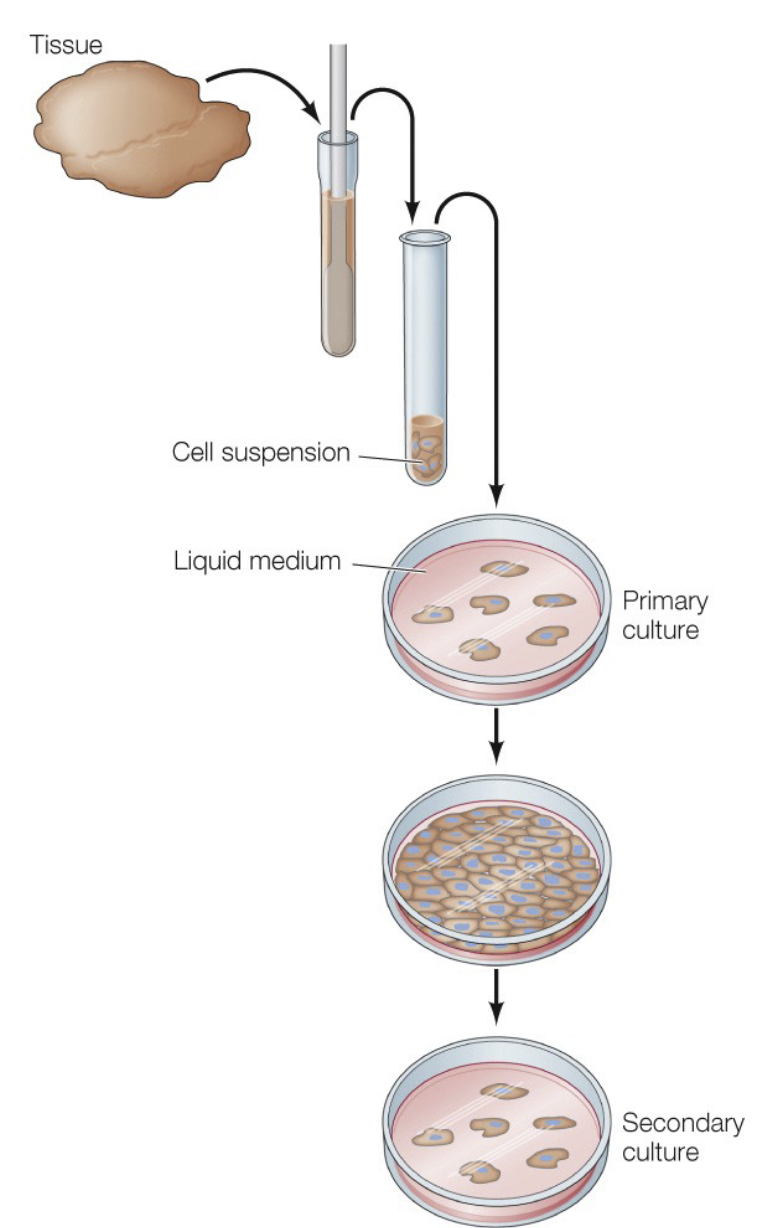
HeLa cell line
Henrietta Lacks, first human cell line derived from a cervical cancer tumor. Important for the development of the polio vaccine and used around the world today.
Viruses
Intracellular parasites that cannot replicate on their own. Smaller and simpler genomes, can be studied in cultured cells. Discovered that some cancers are caused by viruses
How did biomolecules form (3)
Reducing environment, energy, liquid water
What does the RNA World Hypothesis state
The first cell was an enclosed bit of self-replicating RNA in a phospholipid bilayer.
Eventually DNA replaced RNA as the genetic material
Supporting observations for the RNA world hypothesis
Forms spontaneously, RNA can self-replicate, RNA car form enzymes (rRNA, tRNA)
Evolution of Metabolism
The first cells got energy straight from their surroundings. Later, cells started using ATP to store and use energy. O2 built up in atmosphere → cells begin using O2 to make energy more efficiently
Evolution of Cells
Hypothesized that the primordial cell gave rise to 2 varieties of prokaryotes (before nucleus): archaebacteria and bacteria.
Eukaryotes likely arose from an endosymbiosis
Supporting factors: Mitochondria and chloroplasts are prokaryote sized, have their own circular DNA and ribosomes
Covalent bonds
Strongest interaction between atoms
Can form single, double, or triple bonds
Can be polar or nonpolar
What makes carbon special?
Can engage 4 bonds with tetrahedral shape
Double bonds are planar
Can bond with itself to form chains or rings
Can bond with many other atoms
Carbon will form the backbone or organic molecules in living matter
Ionic bonds
Ions held together by attraction of opposite charges
“give and take” of electrons (forms cations and anions)
Hydrogen bonds
Noncovalent bond between a +H and -N/O/F
Weaker than the other bonds but incredibly important in cells.
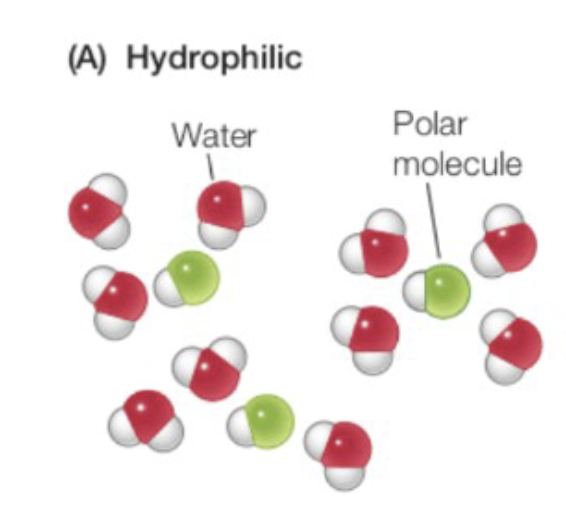
Hydrophilic
Can form hydrogen bonds with water molecules, polar molecules, and surrounds charged ions
Hydrophobic/Lipophilic
Molecules without charged groups are poorly soluble in H2O and associate closely with each other instead
Amphipathic
Molecules having regions with both properties
1st Law: Conservation of ENergy
The total energy of a system and its surroundings is constant
2nd Law: Entropy increases over time
A system will change spontaneously to a state of greater disorder
Catabolic reactions (catabolism)
Downhill reactions. Breakdown of complex molecules into simpler ones and release energy that was used to form them
Anabolic reactions (anabolism)
Uphill reaction. Link simple molecules to form complex molecules. Anabolic reactions require energy to form bonds within the smaller molecules.
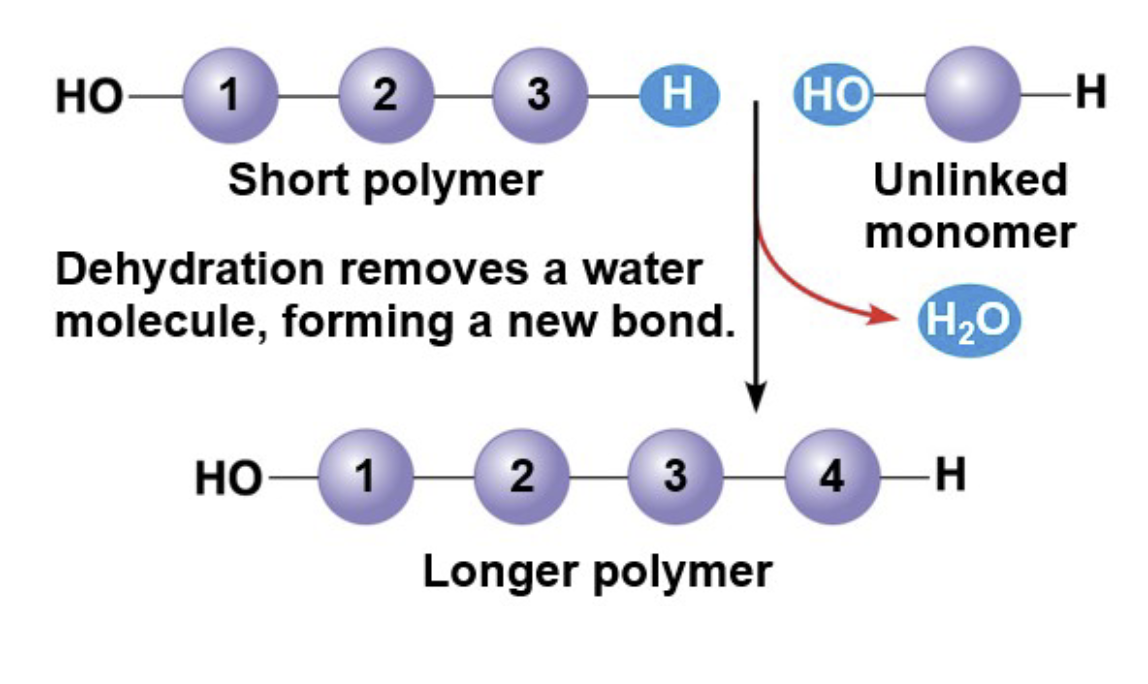
How do polymers form
A dehydration reaction (condensation) that occurs when 2 monomers bond together through the loss of a water molecule.
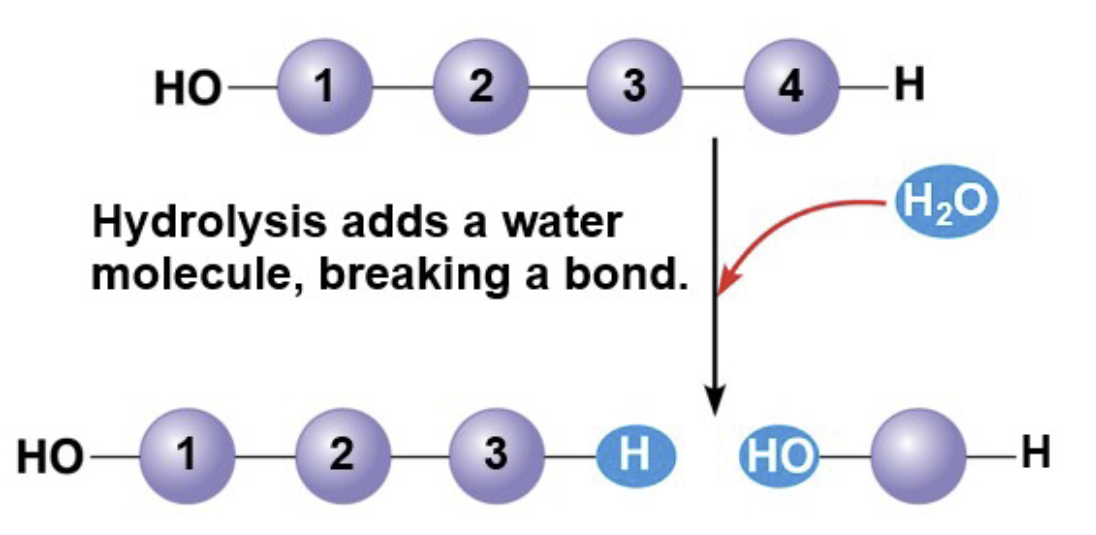
How are polymers broken down
Hydrolysis, a reaction that is essentially the reverse of the dehydration reaction: it needs water
Carbohydrates
Simple sugars, provide energy
Cn(H2O)n
C = 3-7
Name 3 monosccharides
Glucose, fructose, galactose
Oligosaccharide
2-10 monomers
Polysaccharide
more than 10 monomers
What are complex sugar carbohydrates useful for (3)
Energy storage (hydrolyse starch and glycogen)
Structure: cellulose, bacteria can break down.
Signaling: attach to proteins inside cell/on surface like an address
What lipid is used for energy storage and why/how
Triacylglycerides (Triglycerides): 3 fatty acids linked by a glycerol, insoluble in water. Clump together as fat droplets.
Fatty Acids
Fatty acids: long hydrocarbon chains, carboxyl group is polar, Nonpolar C-H bonds
Principle component of cell membranes
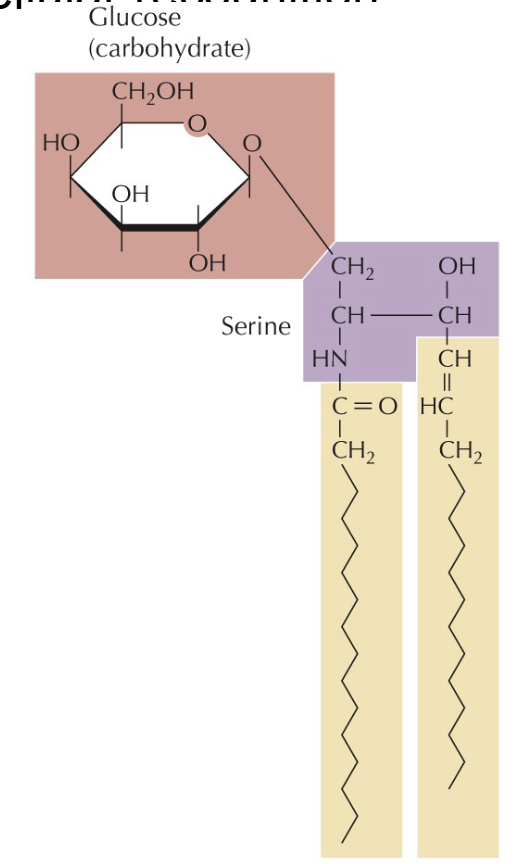
Phospholipids
What are phospholipids
2 C-H chains with a polar head group.
Acyl chains can be held together with glycerol or serine (sphingolipids)
Amphipathic = ideal for cell membranes
What lipids are used for signaling
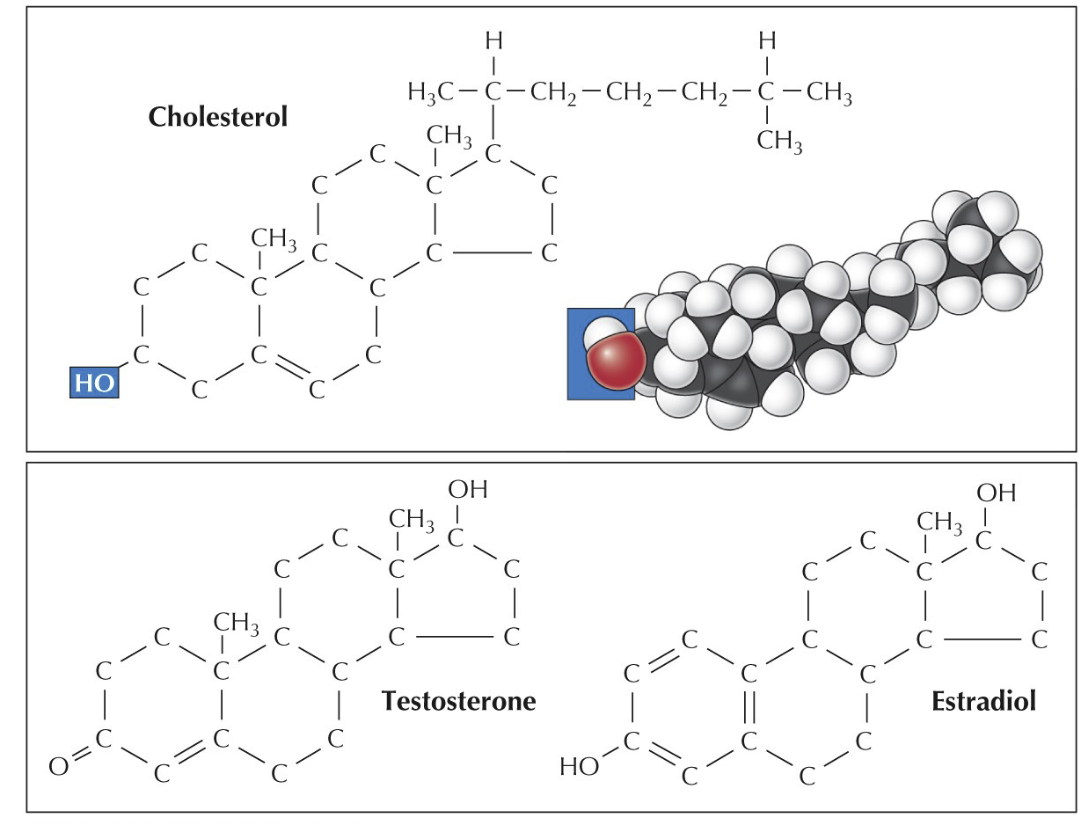
Glycolipids, cholesterol
Glycolipids
Similar to phospholipids, head contains carbohydrate, cellular recognition
Cholesterol
The C/H chain is formed into a multi-ring structure. Also used in hormones (signaling)
end. slide 41 lecutre 2