Topic 12: Buffers
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
What are buffers?
Buffer- a chemical that resists changes in pH when small amounts of acid or base are added
Do buffers stop change in pH?
No
What are acidic buffers made out of?
Weak acid + salt of its conjugate base
What do acidic buffers do in terms of pH?
They resist change in pH to keep solution below pH 7.

Describe the equilibrium position of the weak acid and salt of the conjugate base
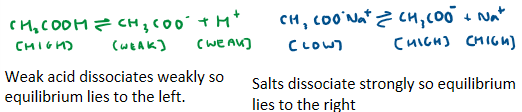
When we add an acid to the acidic buffer, what happens?
H+ ions from acid react with CH3COO- ions in solution (high concentration of negative ions from the salt)
More weak acid (CH3COOH) is produced, which means equilibrium shifts to the left
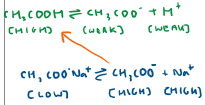
When we add a base to the acidic buffer, what happens?
OH- ions react with H+ ions in solution
There is a low concentration of H+ ions, however, they can be reproduced from a high concentration of CH3COOH to counteract the change
Equilibrium shifts to the right to replace the reacted H+ ions
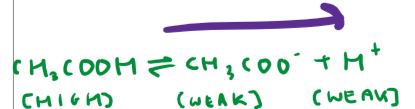
What is another way we can make acidic buffers?
Excess weak acid + strong base
Show an example of this type of acidic buffer and include equations
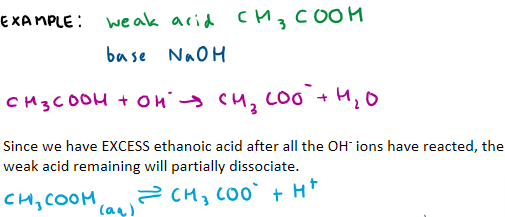
What is a basic buffer made out of?
Weak base and salt of the weak base
What do basic buffers do in terms of pH?
They resist change in pH in order to keep the solution above pH 7.

Write down the reversible reactions for this weak base and it’s salt

When we add a base to the basic buffer, what happens?
The OH- ions react with the NH4+ ions in solution (high concentration of these from the salt).
More NH3 and H2O is produced which means equilibrium shifts to the left.
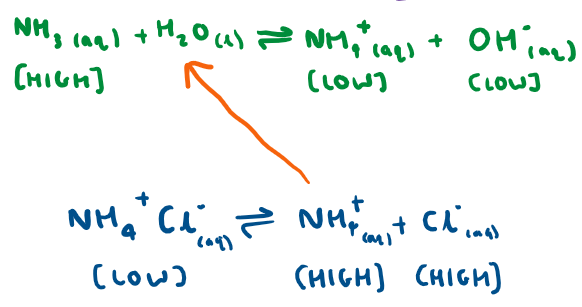
When we add an acid to the basic buffer, what happens?
The H+ ions react with the OH- ions in the solution.
Low concentration of OH- ions, however they can be reproduced from a high concentration of NH3 and H2O to counteract the change.
Equilibrium shifts to the right to replace the reacted OH- ions.
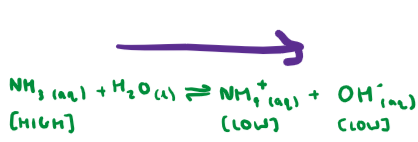
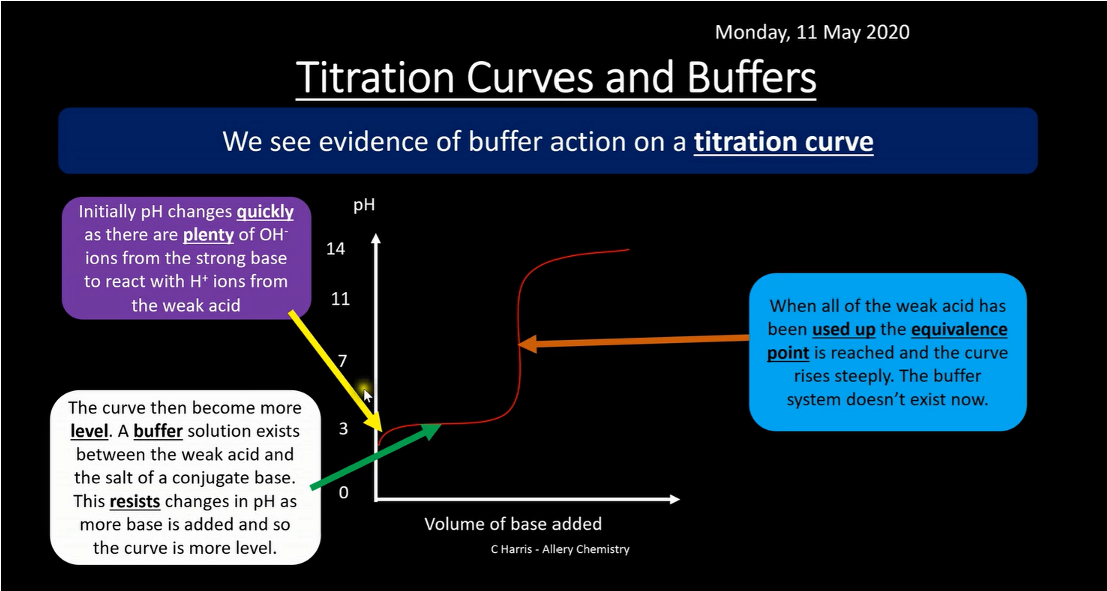
How can you see proof of buffer action on a titration curve?
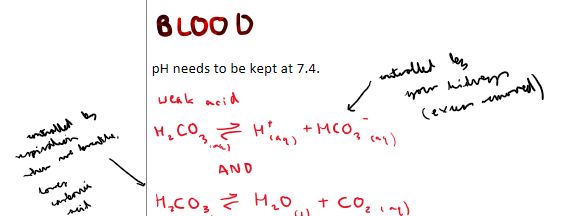
What is the optimal pH for blood?
7.4 pH
What is the equations for blood?