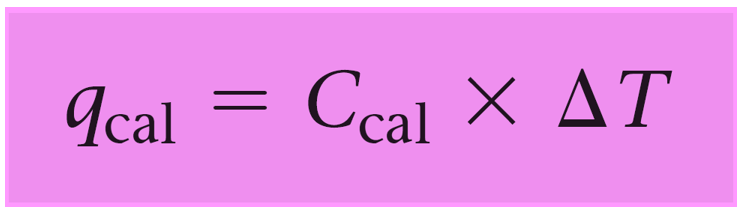Unit 10A - Heat and Thermochemistry - AP Chemistry
1/18
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
system
the region being considered in a problem
surroundings
everything that is outside of the system
heat
the total amount of thermal (kinetic) energy held by the system
units = J, kJ, or cal
temperature
the average thermal (kinetic) energy held by the molecules of the system
units = degrees Celsius or Kelvin
endothermic
heat energy is taken in by the system (feels cold to you, the surroundings)
exothermic
heat is energy is released by the system (feels hot to you, the surroundings)
“positive” heat of a reaction (+q)
heat flows in to the reaction
heat is being pulled out of the surroundings and the temp goes down
“negative” heat of a reaction (-q)
heat flows out of the reaction
heat is being released into the surroundings and the temp goes up
measuring the change in temperature of a solution during dissolving or other physical changes
tells whether or not and how much energy was required/released for that process to occur
measuring the temperature change of a system due to a chemical reaction
tells the amount of energy given off or absorbed during the reaction
equation for quantifying heat energy
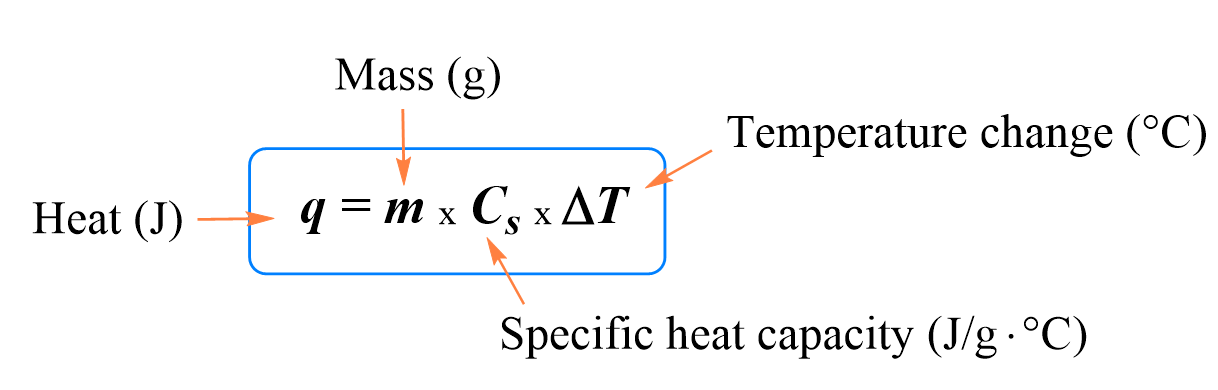
specific heat capacity
a measure of the amount of heat energy required to raise the temperature of 1 gram of a substance 1 degree Celsius
Cp
specific heat capacity measured at constant pressure
for gases, this means the measurement was taken allowing the gas to expand as it was heated
Cv
specific heat capacity measured at constant volume
for gases, this means the measurement was made in a sealed container, allowing the pressure to rise as the gas was heated
calorimetry
the measurement of heat flow
calorimeter
an insulated container for performing reactions that involve measuring heat
coffee cup calorimeter
a calorimeter that is only an insulated container - often containing your system (reactants) dissolved directly in water
can be made from a styrofoam coffee cup
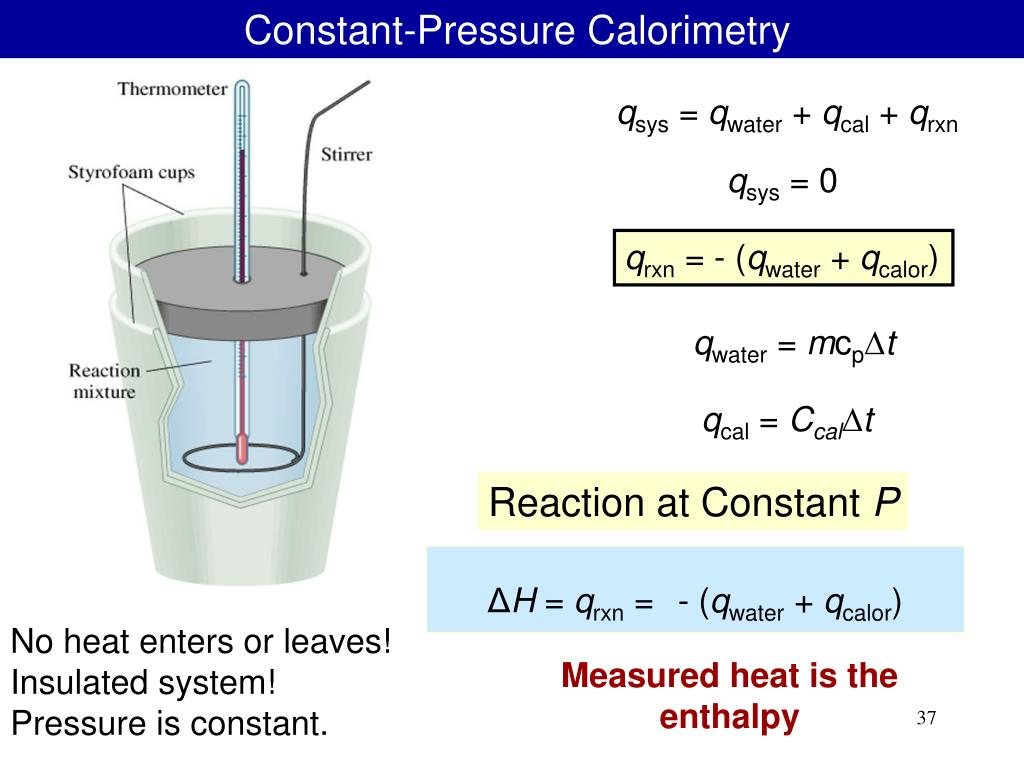
bomb calorimeter
a calorimeter for measuring the heat produced by a chemical reaction
the calorimeter contains a mass of water
the heat from the reaction makes the temp of the water change
calorimeter equation