Week 2: Depression and Anxiety Treatment
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
49 Terms
What was the monoamine hypothesis of depression?
TCAs and monoamine oxidase inhibitors seen as 1st gen of antidepressants, inhibit NA and 5HT3 neurotransmission. It gives a rationale for better drug development beyond these types.
Why was the monoamine hypothesis considered to be inadequate? (4)
1. No full explanation for antidepressant therapeutic action
2. No pathophysiology for depression
3. Doesn't explain why antidepressants take 2-3w to work
4. Doesn't explain why antidepressants are effective in other disorders eg. phobias
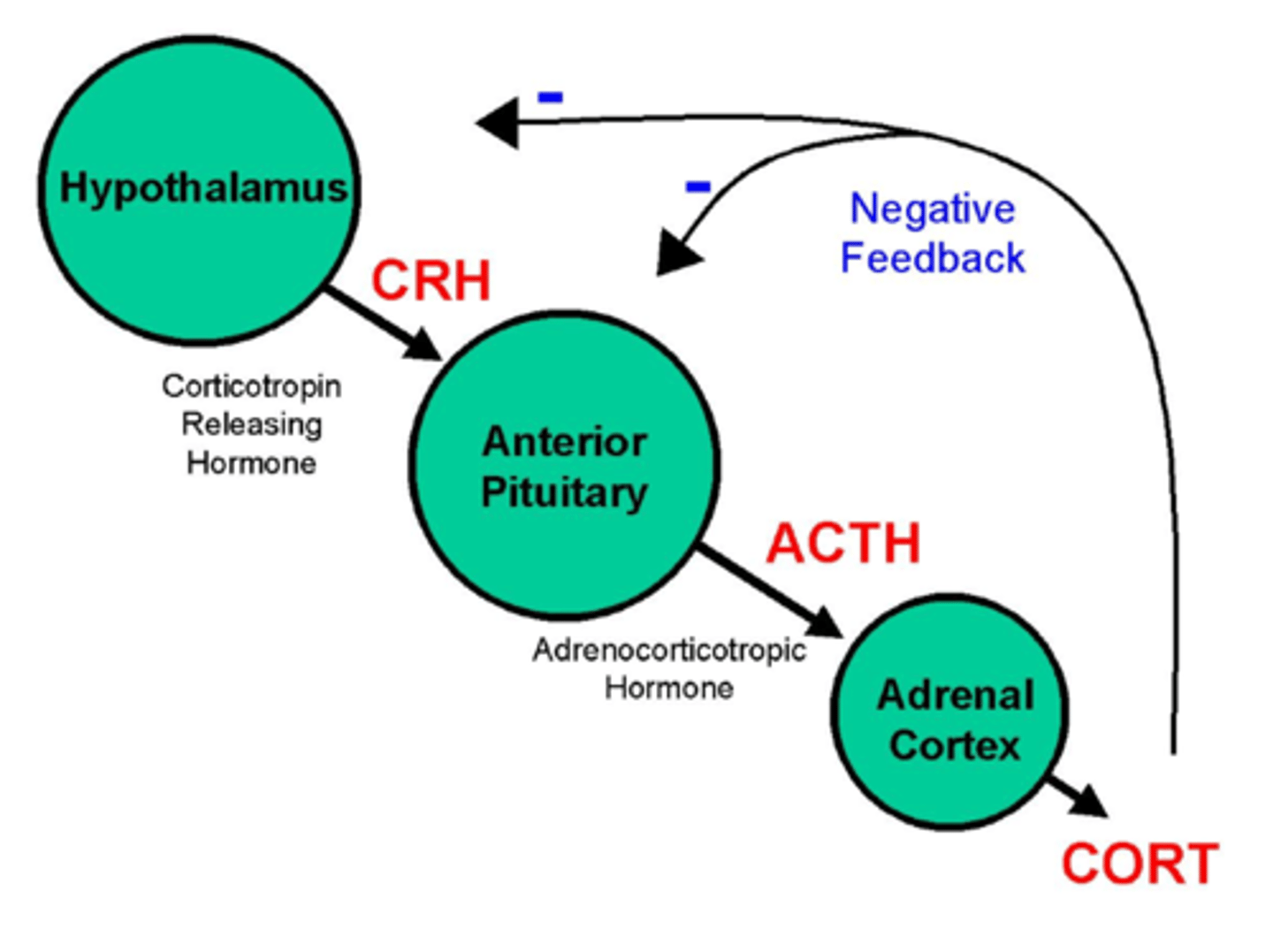
What is the HPA axis in depression?
Depression sees HPA overactivation:
1. High cortisol in saliva, plasma and urine
2. High CRH in CSF and limbic brain region
3. Large size + activity of pituitary + adrenal glands.
There is impaired -ve feedback.
What is a key counselling point in antidepressants?
Effects aren't seen immediately.
How do antidepressants affect the impaired HPA axis?
Enhance -ve feedback and decrease hyperactivity of HPA axis.
What is BDNF?
Brain Derived Neurotrophic Growth Factor - controls neurogenesis, development, dendritic growth, survival + maturation.
What is the neurotrophic hypothesis of depression?
Depression is associated with low BDNF levels. Antidepressants seek to alleviate the depressive behaviour and increase BDNF levels.
What are the neurotransmitters in depression and what do they control? (3)
1. 5-HT - Control anxiety, obsessions and compulsions. Low levels cause depression.
2. NA - Control alertness, anxiety, interest in life. Have a role in reward and stress.
3. DA - Control attention, motivation and reward. Low levels of tyrosine (precursor of DA) cause depression.
What factors do we need to consider for pharmacological mgmt? (4)
1. Adverse effect profile
2. Overdose toxicity
3. Interaction w/ other treatments
4. Cost
etc.
How do you treat mild depression in children and young people (<18)?
Psychological therapy - CBT.
How do you treat moderate-severe depression in children and young people? Outline the care plan. (4)
1. Psychological - for mild cases
2. Combined - add on SSRI (fluoxetine). - for moderate /severe
3. If resistant, alternative psychological therapy
4. If side-effects to fluoxetine - sertraline or citalopram. But fluoxetine has better clinical evidence.
How do you treat mild depression in adults?
1. Psychological intervention
2. NICE doesn't recommend drug treatment unless certain circumstances eg. PMH of moderate-severe depression.
How do you treat moderate-severe depression in adults?
Combined - psychological + anti-depressant.
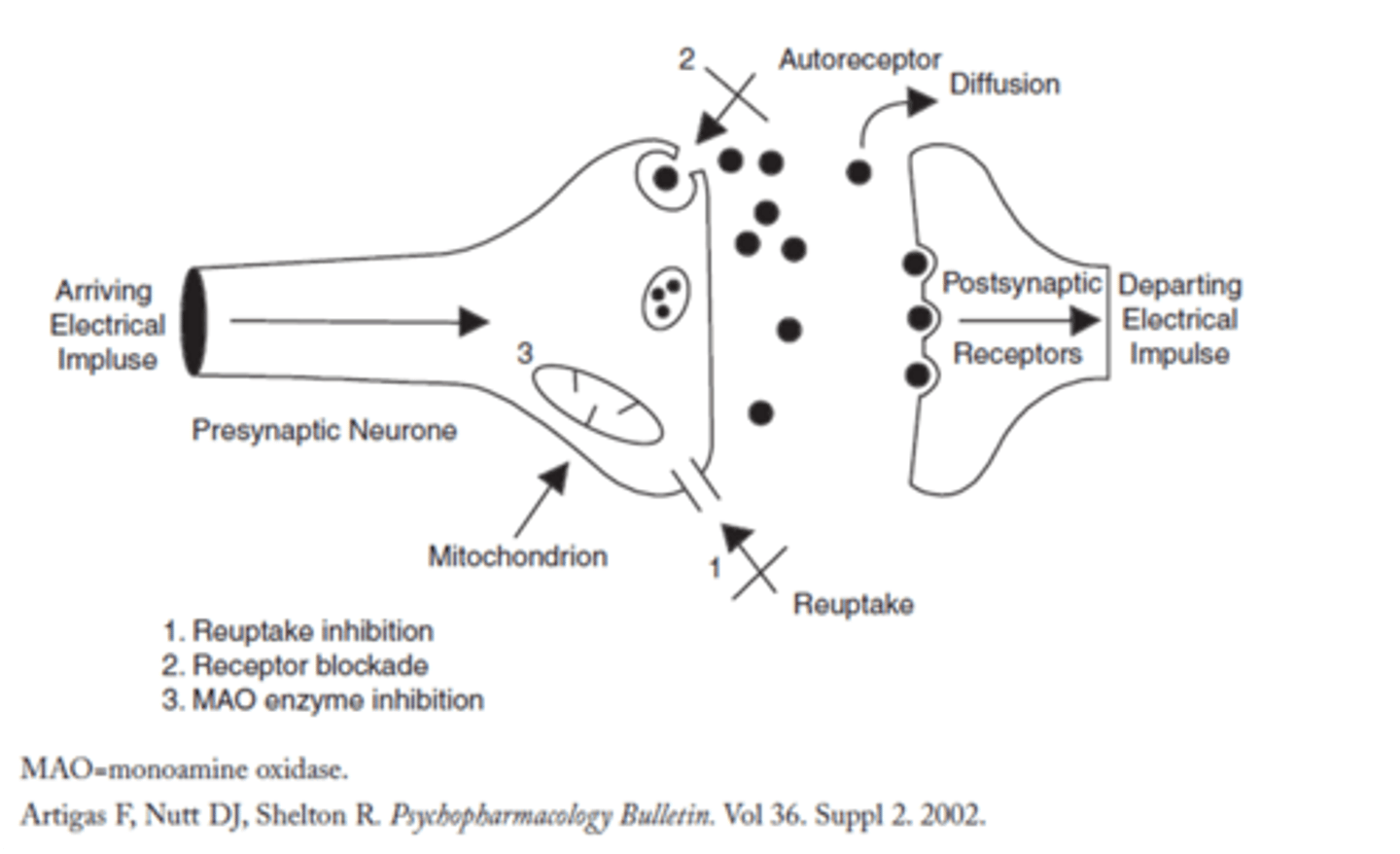
What are the 3 main modes of action of anti-depressants?
1. Inhibit reuptake of neurotransmitter (5HT3)
2. Block receptor (prevent autoreceptors from inhibiting pre/s neurone)
3. Inhibit monoamine oxidase enzyme (on pre/s and cleft, its role is regulating neurotransmitter in pre/s by breaking it down in high amounts).
List the 6 major classes of antidepressants.
1. TCAs.
2. MAOi (monoamine oxidase inhibitor).
3. SSRI (selective serotonin reuptake inhibitor).
4. SNRI (serotonin-noradrenaline reuptake inhibitor) eg. duloxetine, similar to SSRI.
5. NRI (noradrenaline-reuptake inhibitor) eg. reboxetine.
6. Mirtazapine.
How do TCAs work and what are their side-effects? (3)
Inhibit 5HT and NA uptake eg. amitriptyline, imiprapine.
1. Sedative properties (antagonise H1 receptor), which can stop early morning waking but also affect daily function
2. Anti-ACh side effects, i.e. inhibits parasympathetic NS (dry mouth, blurred vision)
3. CV effects since it also block other receptors (fatal in overdose)
What is the cheese reaction with MAOis? Give examples of drugs.
eg. phenelzine, tranylcypromine etc.
Inhibit MOA (which breaks down neurotransmitter), so tyramine (in cheese) isn't metabolised, entering circulatory system causing NA release from peripheral adrenergic neurones (⍺-1), causing a hypertensive crisis due to vasoconstriction.
Avoid tyramine-containing foods.
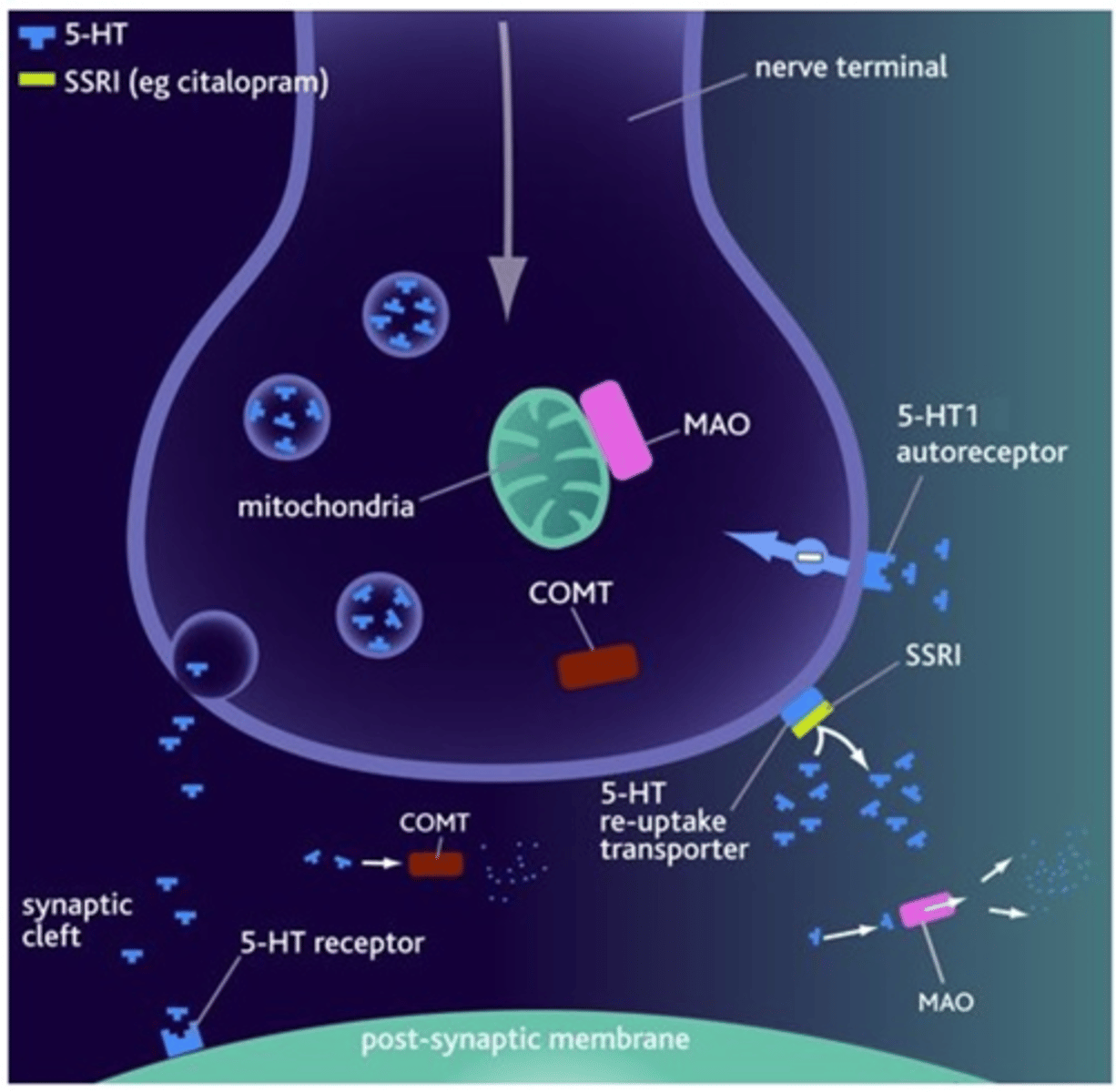
Which anti-depressants are first-line? Give examples and their mechanism.
SSRIs eg. citalopram, fluoxetine, sertraline.
Bind to transporter and block reuptake mechanism so neurotransmitters continue to exist. Has a favourable side effect profile and less toxic in overdose.
What is mirtazapine and how does it work? (2)
An atypical antidepressant that enhances NA and 5HT transmission. It targets:
1. block pre-synaptic ⍺-2 adrenoceptors (autoreceptors) which usually block NA release.
2. block pre-synaptic 5-HT2 receptors, which usually block 5-HT release.
What is the theory for potential delay for clinical effect? What happens with continuous use of antidepressants?
A theory that explains the delayed clinical response for anti-depressants. EG. SSRIs bind to the reuptake transporters, which activate autoreceptors on pre/s neurone, blocking release of 5-HT into the cleft for a period of time.
W/ continuous antidepressant usage, Autoreceptors become desensitised, reinstating release of neurotransmitter.
What is a theory for BDNF explaining clinical effect delay?
Antidepressants elevate BDNF levels, but it takes time for them to be expressed which can explain the clinical effect delay.
Potential delay of clinical effects
2 theories
Monoamine Hypothesis: Antidepressants increase neurotransmission but mood improvement is delayed.
Neurotrophic Hypothesis:
Depression reduces brain-derived neurotrophic factor (BDNF) levels.
Antidepressants elevate BDNF, promoting neurogenesis and synaptic growth.
What is BDNF
Brain Derived Neurotrophic Factor regulates neurogenesis, development, dendritic growth, survival and maturation.
What does the neurotrophic hypothesis of depression propose?
Proposes that depression is associated with reduced brain BDNF levels
Antidepressant treatments alleviate depressive behaviour and BDNF levels
Depression often involves negative emotional biases
Negative views of the self, others, and the world.
Learned helplessness contributes to inactivity and reduced social engagement
What characterises bipolar disorder? What is its incidence?
A cycle between depressed mood and mania.
Depressed mood - 2w+ with core symptoms (low mood and loss of interest), along with 4 other symptoms.
Mania - elevated mood, increased energy, incomprehensible speech, racing thoughts and poor concentration.
1st episode is seen before age 30, peak incidence between 15-19 years.
What is Euthymia
substantial intervening periods in which mood is okay
How can you confirm a BPD diagnosis?
Specialist mental health professionals will diagnose it and make pharmacological recommendations. Has a complicated differential diagnosis.
How can you manage mania in BPD for acute and long-term purposes?
Acute: Antipsychotics (eg. risperidone, haloperidol, olanzapine) and lithium (mood-stabilising drug).
Longer-term: Lithium, valproate (anti-epileptic), quetiapine.
neagtives for Lithium/valporate
Lithium/valporate for longer term mania and bipolar
This requires more frequent blood testing
Narrow therapeutic range
How do you manage bipolar depression? (4)
1. SSRI fluoxetine + olanzapine
2. Quetiapine alone
3. Olanzapine alone (all anti-psychotics)
4. Lamotrigine alone (anti-epileptic/anti-convulsant).
Should check guidelines if pt. is taking lithium.
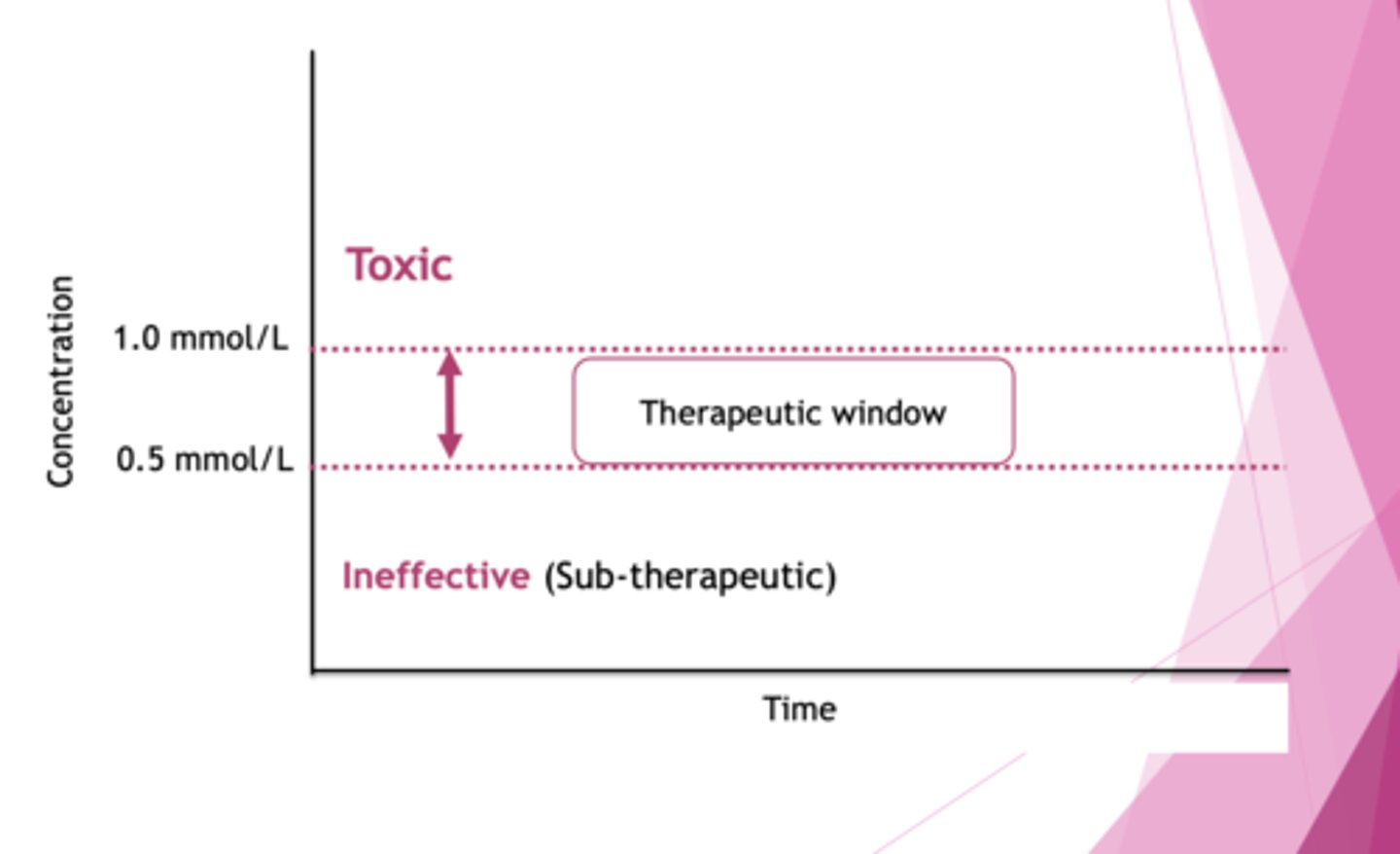
How do you monitor therapeutic doses of lithium?
Narrow TW (0.5 mmol/L). If you exceed by 1mmol/L, there is toxicity and going subtherapeutic renders it ineffective.
Toxicity can cause diarrhoea, drowsiness, weakness, convulsions etc.
What is generalised anxiety disorder?
Worry for >6mo disproportionate to stress, w/ insomnia, muscle tension w/ headaches, backaches and autonomic physical symptoms (palpitations, HR).
What are the causes of anxiety? (6)
1. Genetics (1º relative)
2. Neurochemical + neurohormonal (HPA axis - ↑cortisol)
3. Environmental
4. Drugs + meds
5. Physical and mental health
6. Diet
What are the 4 aspects of neurobiology regarding GAD (Generalised Anxiety Disorder)?
1. Neuroimaging
2. Genetics (nature v nurture)
3. Neurotransmitters
4. Neuroendocrine (HPA)
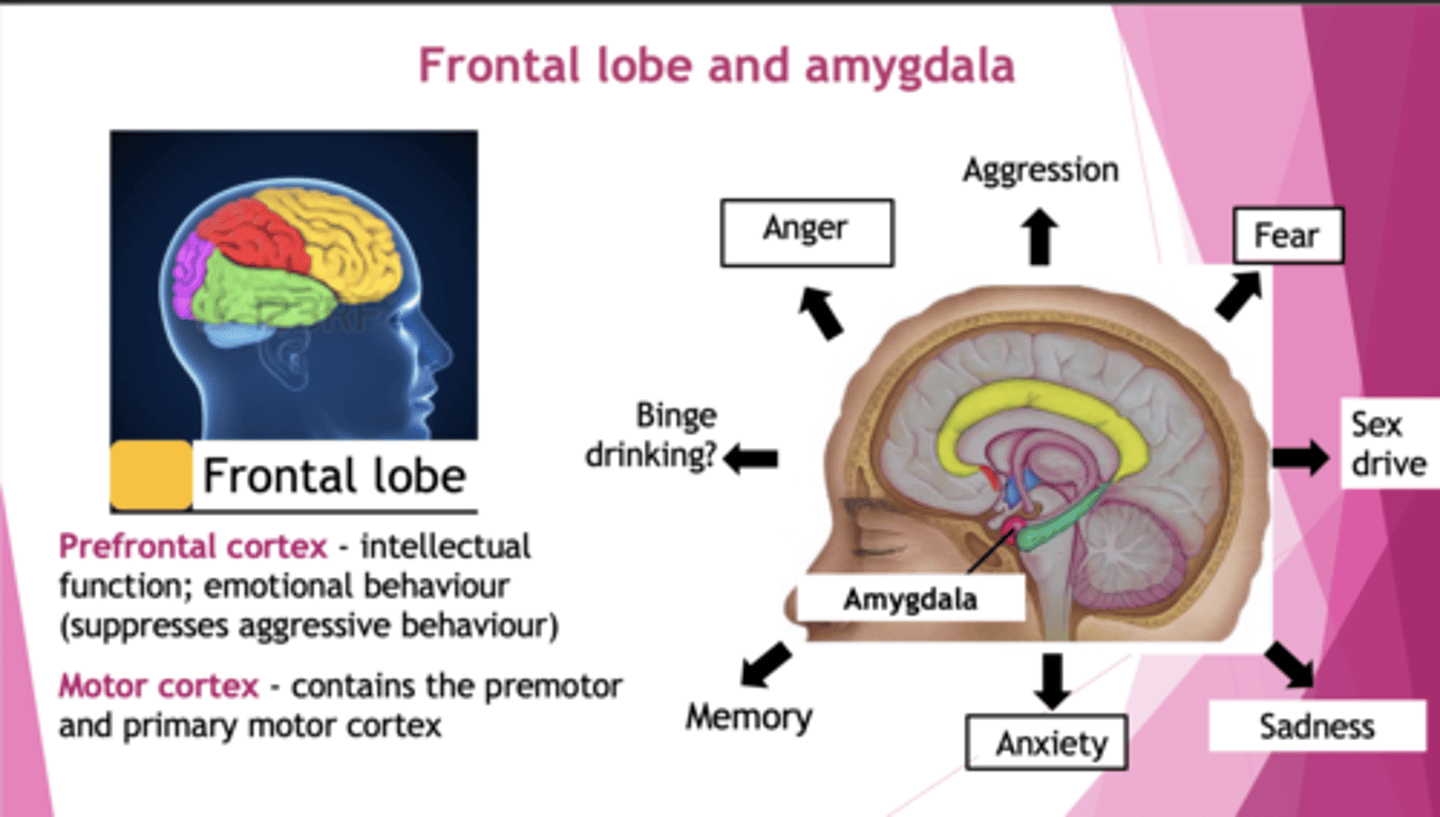
Explain the neuroanatomy of anxiety
The amygdala = triggers fight/flight + acute threat response
The bed nucleus of the stria terminalis = handles uncertainity + chronic threat response
Frontal lobe = uses logic to regulate emotional centres
What do the prefrontal cortex and motor cortex control?
Prefrontal: intellectual, emotional behaviour (suppresses aggression). Connected to amygdala.
Motor: contains premotor and primary motor cortex.
How does GAD appear in neuroimaging?
Decreased connectivity between amygdala and prefrontal cortex.
How is GAD linked to genetics?
Genes - 30-50%.
Environment - 50-70% (eg. trauma, stressful home life contribute more than genetics).
What are the neurotransmitters associated with GAD? (4)
1. GABA - GABA(A) is downregulated so less inhibition (more excitation), treated with GABA agonists.
2. 5-HT - SSRIs have efficacy for GAD (↑serotonin levels help)
3. CRH - Unsuccessful clinical trials.
4. Neuropeptides - hypersensitive to CCK agonists. Antagonists were unsuccessful in trials.
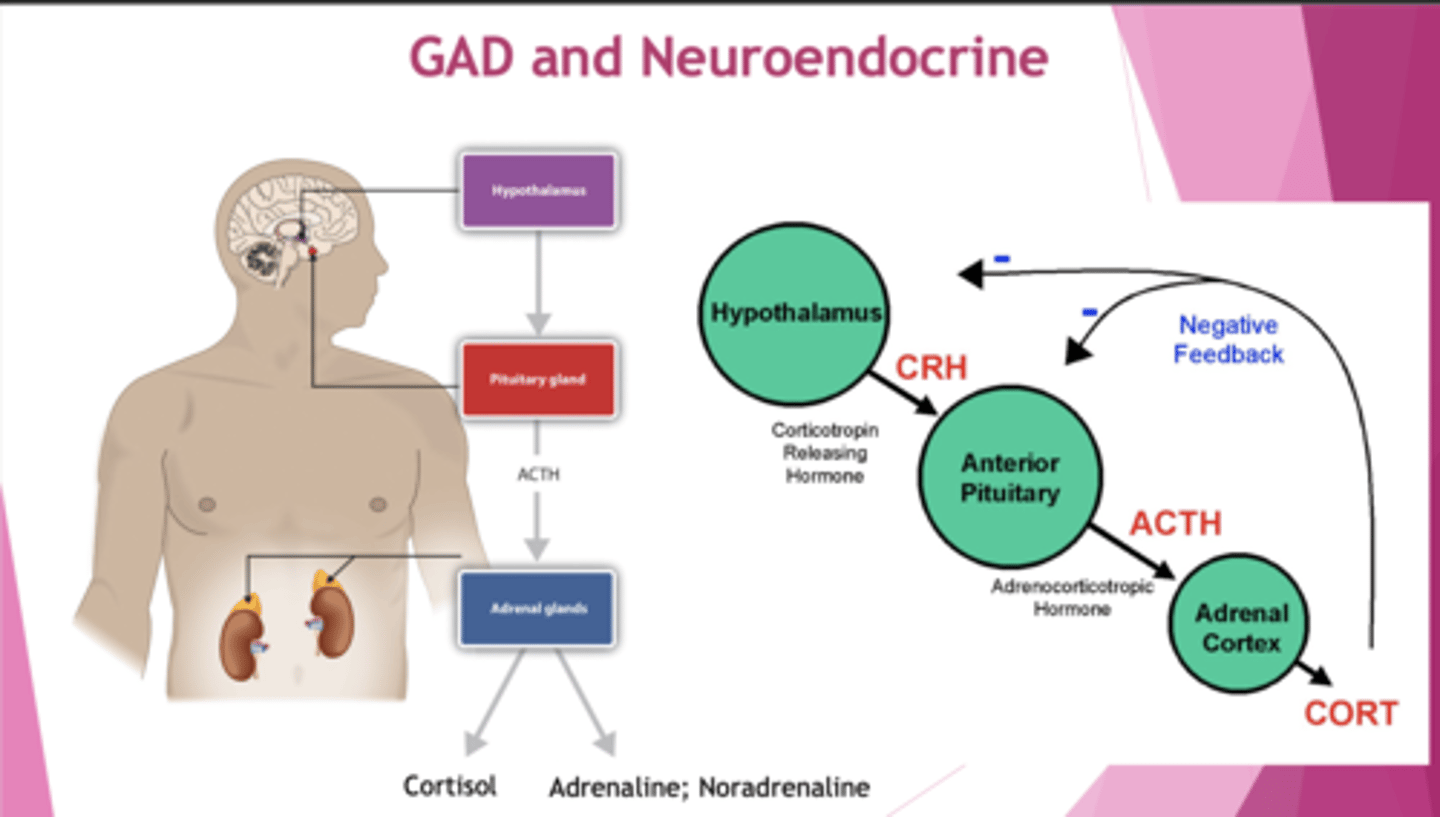
How is GAD linked to the HPA axis?
↑HPA axis, ↑cortisol, ↑NA and A. Overstimulation of SNS.
How do you treat the autonomic symptoms of GAD? What is a counselling point with the drug?
i.e. SNS overactivity (eg. palpitations and tachycardia).
Target with β-blockers (propranalol) which reduce autonomic effect.
Note: don't withdraw abruptly to prevent rebound effects.
How do you treat the anxiety symptoms of GAD? (3)
1. Psychological interventions
2. Offer SSRI (1st line is sertraline).
3. Do not offer benzodiazepine (anxiolytic) except for short-term use during a crisis. They target GABA(A) but can cause dependence and tolerance issues.
What are benzodiazepines (BDZ) used for? (6)
Target GABA(A) receptor complex.
1. Muscle relaxation
2. Insomnia (anxiolytic effect for hypnosis)
3. Pre-meds (treat anxiety before major surgery)
4. Epilepsy
5. Alcohol withdrawal
6. Anxiety
What are some other Pharmacological Treatments for anxiety disorder
Pregabalin (GAD; risk of misuse). - risk if taken with opioids/alcohol , helpful for short term
Propranolol (physical symptoms by the SNS of anxiety; toxic in overdose).
Antipsychotics (PTSD re-experiencing symptoms).
Clomipramine (OCD).
Buspirone (GAD, slower onset). - causes Gi issue
Quetiapine (sedation, weight gain)
How are BDZs classified? (3)
By their t-1/2.
1. Short-acting: less than 5hrs. For hypnosis purposes.
2. Short-intermediate: 5-24hrs.
3. Long-acting: >24hrs.
The classification influences their use eg. hypnotic drugs have a shorter t-1/2.
Explain the GABA(A) receptor, its subunits and what binds. (4)
GABA has 5 units - 2 ⍺, 2 β and 1 gamma.
1. BDZ bind between ⍺ and gamma subunits.
2. GABA binds between ⍺ and β subunits.
3. Alcohol will bind to ⍺ subunits.
4. Neurosteroids (anaesthetics) and barbiturates bind to β subunits.
Binding opens Cl- ion pore, causing Cl- influx.
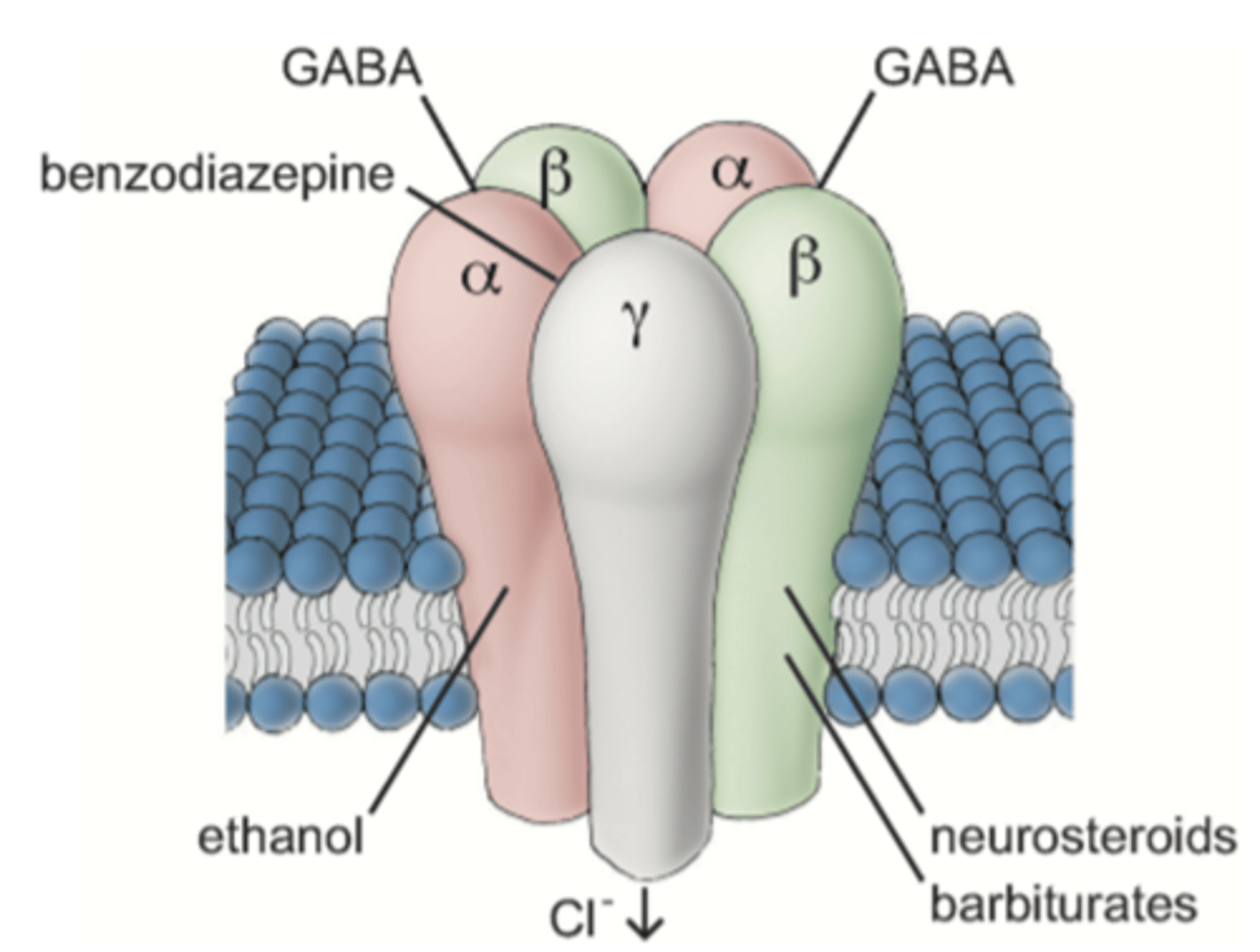
Explain the MOA of BDZs. (3)
1. BDZs are +ve allosteric modulators on the GABA(A) complex (btwn ⍺ and gamma subunits). Cause a conformational change that allows GABA to bind (btwn ⍺ and β subunits).
2. When GABA binds, receptor undergoes another conformational change, opening Cl- channel pore.
3. Causes Cl- influx into neurone, causing hyperpolarisation → inhibiting neurotransmission.
Promotes inhibition of excitability.
What should you prioritise treating with anxiety and depression?
The depression first, then anxiety symptoms.