Chapter 2 - Chem
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
Classical Laws: Law of Conservation of Mass (Chapter 2)
Mass/matter cannot be created or destroyed
Mass at beginning and end of a chemical reaction must be the same
Classical Laws: Law of Definite Proportions (Constant Composition) (Chapter 2)
Compounds are composed of a definite (fixed) proportion by mass of their elements
Use percent by mass of a compound to determine how much of each element a certain sample would contain
Classical Laws: Law of Multiple Proportions (Chapter 2)
If multiple compounds contain the same elements, the ratio of masses of them will be small whole numbers
Can compare ratios of masses to determine chemical formulas
Definite Proportions: Percent Composition (Chapter 2)
If given masses of each element in a compound and mass of total compound, can find ratios or precents of each element in compounds
Ex.
A sample of CO2 contains 47.13 g C and 125.57 g O. What is the percent by mass of each element?
47.13 + 125.57 = 172.70 g CO2
47.13 g C/172.70 g CO2 × 100% = 27.29% C
125.57 g O/172.70 g CO2 × 100% = 72.71% O
Percent Composition Practice (Chapter 2)
A sample of CO2 contains 47.13 g C and 125.57 g O. What is the percent by mass of each element?
47.13 + 125.57 = 172.70 g CO2
47.13 g C/172.70 g CO2 × 100% = 27.29% C
125.57 g O/172.70 g CO2 × 100% = 72.71% O
Dalton’s Atomic Theory (Chapter 2)
Summarizes the classical laws:
Matter is made of indivisible atoms (not quite true, they are made of subatomic particles)
Each atom is identical to others of the same element but different from other elements (not quite true, isotopes and ions exist)
Atoms combine in whole number ratios to form compounds
Some atoms of the same elements can combine in different whole number ratios to form different compounds
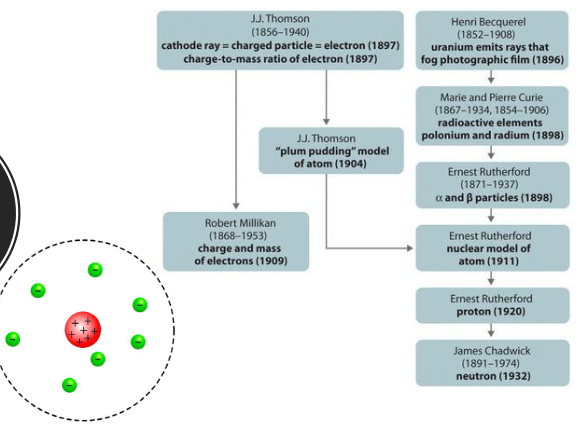
History of the Atom (Chapter 2)
JJ. Thomson (1856-1940)
Cathode ray = charged particle = electron (1897)
Charge-to-mass ratio of electron (1897)
“Plum pudding” model of atom (1904)
Robert Millikan (1868-1953)
Charge and a mass of electrons (1909)
Henri Becquerel (1852-1908)
Uranium emits rays that fog photographic film (1896)
Marie and Pierre Curie (1867-1934, 1854-1906)
Radioactive elements
Polonium and radium (1898)
Ernest Rutherford (1871-1937)
a (alpha) and B (beta) particles (1998)
Nuclear model of atom (1911)
Proton (1920)
James Chadwick (1891-1974)
Neutron (1932)
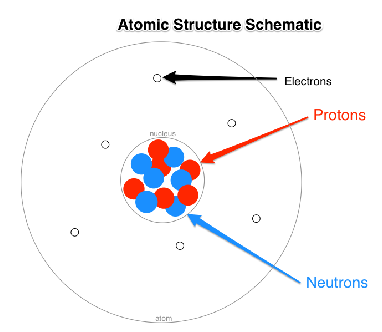
Subatomic Particles (Chapter 2)
Make up atoms
Protons and Neutrons are inside the nucleus
Electrons are outside of the nucleus but within the atom
Proton:
Relative charge = +1
Relative mass (amu) = 1
Neutron:
Relative charge = 0
Relative mass (amu) = 1
Electron:
Relative charge = -1
Relative mass (amu) = 0
Mass Number & Particles (Chapter 2)
Mass number: rounded atomic mass (decimal umber) from periodic table
Sum of protons and neutrons
Atomic number: whole number from periodic table
Number of protons (also electrons if neutral)
Example: sodium
Mass number: 23
Atomic number: 11
Protons & electrons: 11
Neutrons: 12 (23-11)

Mass Number Practice (Chapter 2)
Sodium
Mass number:
Atomic number:
Protons & electrons:
Neutrons:
23
11
11
12 (23-11)
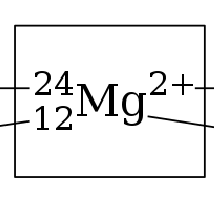
Atomic Symbols (Chapter 2)
Mass number: 24 (12 protons +12 Neutrons)
Atomic number: 12 (12 protons)
Charge: 2+ (12 protons - 10 electrons)
Chemical element: Mg (Magnesium)

Particle & Atomic Symbols Practice (Chapter 2)
Tungsten mass number, atomic number, protons, neutrons, electrons
Atomic symbol for atom with 15 protons & 16 neutrons
How many protons, neutrons, & electrons in 73/32 Ge?
184 mass, 74 atomic
74 protons & electrons
184-74 = 110 neutrons
31/15 P
p+ = 32, n0 = 41, e- = 32
Ions (Chapter 2)
Ions are charge particles that result from an atom’s loss or gain of electrons
Use number of protons and electrons to determine charge
Remember: electrons are negative
Example:
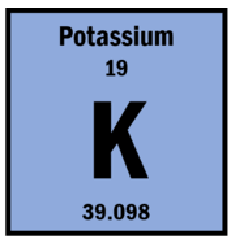
A potassium ion contains 18 electrons, what is its charge?
K has 19 protons if more protons than electrons (electron lost), +1
Ions Practice (Chapter 2)
An ion contains 16 protons, 16 neutrons, and 18 electrons. What element is it an ion of and what is its charge?
A magnesium ion contains 10 electrons, what is its charge?
S is atomic number 16, if more electrons than proton (electrons gained), charge is negative, -2
Mg has 12 protons, +2
Average Atomic Mass (Chapter)
Mass on periodic table is weighted average of the mass of all atoms of that element
Elements have isotopes- atom with different number of neutrons
Ex: C - 12 has 6 neutrons while C - 14 has 8 (number after dash = mass number)
Can use actual masses of isotopes (not quite whole numbers0 and their relative abundances (usually as precentages0 to calculate average atomic mass
The mass or precent of an isotope could also be calculated if given the average atomic mass

Calculate Average Atomic Mass Example (Chapter 2)
Percents are unknown, but together add up to 100%
Answer

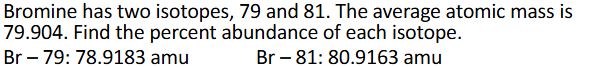
Average Atomic Mass Practice (Chapter 2)
Answer
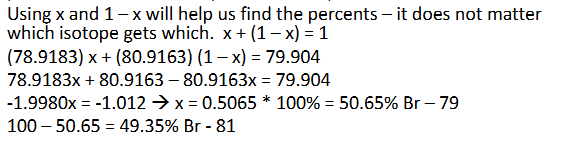
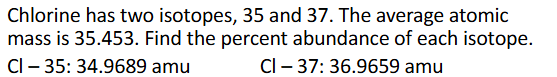
Average Atomic Mass Practice Pt.2 (Chapter 2)
Answer
Periodic Table Development (Chapter 2)
Mendeleev & Meyer independently developed ways to organize elements based on physical properties
Mendeleev gets most of the credit because he left gaps for where undiscovered elements should go - and they did, once discovered
Period Table Organization Pt.1 (Chapter 2)
Column: Group
Row: Period

Period Table Organization Pt.2 (Chapter 2)
Group numbers 1-18 for all, 1A-8A for representative elements, 1B - 8B for transition elements
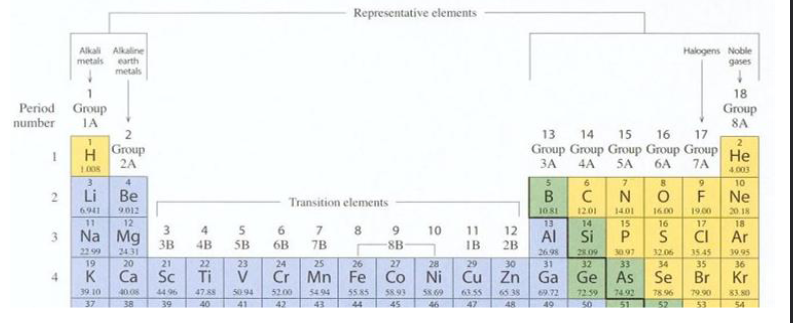
Periodic Table Organization Pt.3 (Chapter 2)
Top left: Nonmetals
Metalloids: Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, Astatine
Right side/Middle: Metals
Row 4 - 7/Column 3 and so on: Transition Metals
Bottom section: Inner-Transition Metals
Main Group: 1, 2, 13, -18
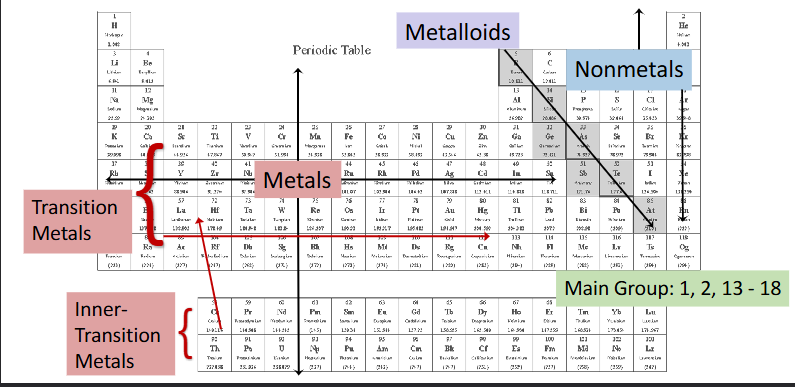
Types of Elements (Chapter 2)
Metals
Metalloids
Nonmetals
Metals (Chapter 2)
Usually solid at room temperature, shiny, malleable, ductile, good conductors of heat & electricity, ex. copper, sodium, silver
Metalloids (Chapter 2)
Properties of both metals & nonmetals, semiconductors, ex. germanium, arsenic, boron
Nonmetals (Chapter 2)
May be gas, liquid, or solid at room temperature, brittle, poor conductors, ex. sulfur, nitrogen, bromine
Alkali Metals (Chapter 2)
Group 1 excluding (Hydrogen)
Very reactive
Very soft
Make water have a high pH Alkaline (Basic)
Alkaline Earth Metals (Chapter 2)
Group 2
Reactive
Soft
Make water alkaline (high pH Base)
Halogens (Chapter 2)
Group 17
Nonmetals & metalloids
Very reactive
2 gases, 1 liquid, 2 solids at room temperature
Not found in nature very easily
Noble Gases (Chapter 2)
Group 18
Nonmetals
Nonreactive (very stable)
Gases
Found in nature very easily