Chemistry Exam Review - ALL UNITS (2-6)
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
Describe atomic radius
The size of the atom
Atomic radius INCREASES down a group
More orbits = electrons are farther from nucleus (BIGGER ATOMIC RADIUS)
Atomic radius DECREASES across a period
More protons = STRONGER PULL on ELECTRONS (SMALLER ATOMIC RADIUS)
Describe effective nuclear charge
Effective Nuclear Charge (ENC) exerts a pull on the valence electrons
ENC = # protons - # core electrons
THINK “more protons = more ENC”
HIGHER ENC
= STRONGER attraction of valence electrons
= SMALLER atomic radius
Describe the ionic radius of cations
Positive Ions (Cations)
LOSE electrons
Lose 1 energy level/shell
Radius DECREASES
Cations → LOSE electrons → Fewer orbits
= SMALLER ionic radius
Describe the ionic radius of anions
Negative Ions (Anions)
GAIN electrons
Radius INCREASES due to electrons repelling each other (think: it needs to “make room” for extra e-)
Anions → GAIN electrons → Electron repulsion
= LARGER ionic radius
Describe the Reactivity of METALS
Metals react by LOSING electrons
Metal reactivity INCREASES down a group
Valence electrons FARTHER from the nucleus (more shielded) = EASIER TO LOSE
Metal reactivity DECREASES across a period
# of valence electrons INCREASES = more valence electrons need to be given away (takes more energy to do this - HARDER TO LOSE)
# of protons increases = MORE attraction of electrons (HARDER TO LOSE)
Describe the Reactivity of NONMETALS
Nonmetals react by GAINING electrons
Nonmetal reactivity DECREASES down a group
Valence electrons are FARTHER from the nucleus (more shielded) = HARDER TO GAIN
Nonmetal reactivity INCREASES across a period
# of valence electrons INCREASES = fewer valence electrons need to be gained (takes less energy - EASIER TO GAIN)
# of protons increases = MORE attraction of electrons (EASIER TO GAIN)
describe electron shielding
outer (valence) electrons are partially shielded from the attractive force of the protons in the nucleus by the orbits in between the nucleus and the valence shell
More orbits = more shielding = e- lost more easily
Describe ionization energy
Ionization Energy (IE) - amount of energy required to remove an electron from the atom or ion (in gaseous state)
X(g) + energy → X+ (g) + e-
How hard it is for an atom to lose an electron (harder = ↑ IE)
Ionization energy INCREASES as you move left to right ACROSS a period
MORE PROTONS
= higher ENC
(effective nuclear charge)
= smaller atomic radius
= MORE energy needed to remove an electronIonization energy DECREASES as you move DOWN a group
MORE ELECTRON SHELLS/ORBITS
= electrons farther from nucleus
= electron shielding
= LESS energy needed to remove an electron
Describe second ionization energy
2nd Ionization Energy (2nd IE) - amount of energy required to remove a SECOND electron from an ion (in gaseous state)
It is always harder to remove a 2nd electron
Removing 1st electron DECREASES
atomic radiusFewer electrons repelling each other & stronger pull from protons
Takes MORE ENERGY to remove a 2nd electron
Describe electron affinity
lectron Affinity (EA) - The energy RELEASED from a (gaseous) atom ACCEPTING an electron
How much an atom wants to gain an electron
Inversely related to atomic radius
Electron affinity INCREASES as you go left to right across a period
SMALLER atomic radius
= electrons closer to nucleus
= easier to gain/attract electrons
Electron affinity DECREASES as you go down a group
MORE ELECTRON SHELLS/ORBITS
= electrons farther from nucleus
= more shielding
= harder to gain/attract electrons
Describe electronegativity
Electronegativity (EN) - the tendency for an element to attract shared electrons in a chemical bond
How strongly an element pulls the electrons to their side of the bond
HIGHER EN means MORE electron attraction for an element
Electronegativity INCREASES as you go left to right across a period
SMALLER atomic radius
= electrons closer to nucleus
= more pull on the electrons in a bonds
Electronegativity DECREASES as you go down a groupMORE ELECTRON SHELLS/ORBITS
= electrons farther from nucleus
= more shielding
= less pull on the electrons in a bond
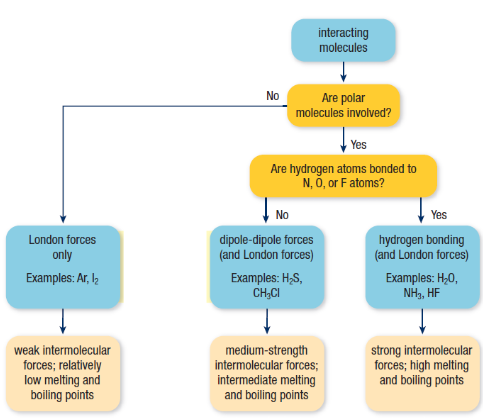
Intramolecular vs. Intermolecular forces
INTRAmolecular forces - attractive forces WITHIN a molecule
TIP - think “intramurals” (within a school)
Ionic, polar, or nonpolar
INTERmolecular forces - attractive forces BETWEEN molecules
TIP - think “interact” (with others)
The stronger the intermolecular force,
the higher the melting/boiling point
The type of intramolecular force (ionic vs. covalent) determines
the type of intermolecular force
Types of intermolecular forces
London Dispersion
Weakest force
Between ALL molecules (polar and nonpolar)
Dipole-Dipole
Pretty strong
Forces between ONLY polar molecules
The greater ΔEN the stronger the dipole-dipole forces
Hydrogen Bonding
Strongest intermolecular force
Generally occurs when hydrogen bonds with N, O, or F (makes an extremely polar bond; very high ΔEN)
REMEMBER - H=NOF
Mass of subatomic particles
Protons = 1
Neutrons = 1
Electrons = less than 1
What are the products of a decomposition reaction where a HYDRATE is the reactant?
Ionic compound + water (dehydration)
What are the products of a decomposition reaction where a METAL NITRATE is the reactant?
metal nitrite + oxygen
What are the products of a decomposition reaction where a METAL CARBONATE is the reactant?
Metal oxide + carbon dioxide
What are the products of a decomposition reaction where a METAL HYDROXIDE is the reactant?
Metal oxide + water
What does a nonmetal oxide + water form?
Acid
What does a metal oxide + water form?
Base
What are the prefixes and suffixes for derived acids?
Per ——- ic acid = 1 more oxygen
--ous = minus 1 oxygen
hypo —— ous = Minus 2 oxygen
Name the strong acids and bases
Acids - HI, HBr, HCl, H2SO4, HNO3, HClO3
Bases - Group 1 and 2
Boyle’s Law
Volume and temp inversely related
P1V1 = P2V2
Charle’s law
Volume and temperature are directly related
V1 = V2
T1 T2
Gay-Lussac’s Law
Pressure and temp are directly related
P1 = P2
T1 T2
Combined gas law
P1V1 = P2V2
T1 T2
Ideal Gas law
PV = nRT
Avogadro’s law
Equal volumes of gases have equal number or molecules and moles of molecules
V x mole ratio
Molar Volume
STP = 22.4 L/mol
SATP = 24.8 L/mol
n = V / Vm
What does a metal plus water make?
Metal hydroxide + hydrogen