C2 - Bonding, Structure and The Properties of Matter
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
71 Terms
What are the types of atom bonds?
ionic
covalent
metallic
What are the characteristics of ionic bond?
metals and non-metals
electron transfer occurs, forming ions
oppositely charged ions attract through electrostatic attraction
What are the characteristics of covalent bond?
non-metal atoms
share a pair of electrons
What are the characteristics of metallic bond?
occurs in metals and metal alloys
delocalised electrons hold 2 metal ions together
What is the negative ion name and how have them become like this?
called anions, have gained electron(s)
What is the positive ion name and how have them become like this?
called cations, have lost electron(s)
What is the outer shell and outer electrons called?
valance shell and valance electrons
How to describe an ionic bond - Method
State how many electrons the metal has lost and its new charge.
State how many electrons the non-metal has gained and its new charge.
State that oppositely charged ions are held together by strong forces of electrostatic attraction in ionic bonds.
Electrostatic forces are…
…strong, acting in all directions and so they form the basis of an ionic bond.
What is the arrangement of lattices formed by ionic compounds?
A regular arrangement of alternating positive and negative ions which the ions are tightly packed together. Strong electrostatic forces hold the lattice together.
Why do ionic lattice compounds have high melting andf boiling points?
As a result of so many electrostatic forces existing in this lattice structure.
What is a molecule?
2 or more atoms covalently bonded together.
What are the strengths of the bonds in covalent bonding? (paragraph)
They have very strong covalent bonds to hold atoms together in a molecule, but the intermolecular forces between individual molecules is very weak. This is why simple covalent structures can be easily seperated without breaking bonds but giant covalent lattices involve breaking bonds when separating as molecules are bonded together bu covalent bonds.
Different names of electrons - Covalent Bonds
electrons on the valance shell wjich atre not involved in the covalent bond are non-bonding electrons
electrons which are shared are bonding electrons
Examples of simple covalent molecules:
O2
H2
Cl2
N2
HCl
H2O
NH3
CH4
N2 Bond Diagram - Dot and Cross
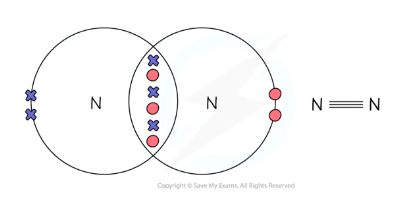
Examples of large Covalent molecules / structures
polymers
giant covalent structures
Examples of polymers with Covalent Bonds
polythene - used in plastic bags
polyvinyl chloride (PVC) - has many industrial applications, mostly water pipes
Advantage and Disadvantage of Dot and Cross Diagram
Useful for illustrating the transfer of electrons
fails to illustrate 3D arrangement of atoms
Advantage and Disadvantage of Ball and Stick Diagram
useful for visualizing shape of the atom
shows large gaps in between atoms, which in reality is not the case as gaps are miniscule
Advantage and Disadvantage of 2D Representation of Molecule Diagram
indicate which atoms are in molecule and how they’re connected in a simple way
fail to illustrate relative size of atoms and bonds
Metals consist of…
…giant structures of atoms arranged in a regular pattern.
How is a metal lattice formed?
Each metal atom loses its valance electrons, which become delocalised. This means there are negative electrons and positive ions in the lattice. The negative electrons are attracted to the positive ions and vice versa, so they’re all bonded to form a lattice. Individual metal atoms are held together by strong metallic bonds forming a lattice structure.
Why can metals carry charge?
Because they have delocalised electrons which are free to move in the spaces between metal atoms’ nuclei.
Description of movement in the 3 states.
Solid: vibrate around a fixed position
Liquid: move around each other
Gas: move quickly in all directions
Description of arrangement in the 3 states.
Solid: regular arrangement
Liquid: random arrangement
Gas: random arrangement
Description of particle distance in the 3 states.
Solid: very close together, tightly packed
Liquid: fairly close
Gas: far apart
The amount of energy needed to change the state from solid→liquid or liquid→gas depends on…
…the strength of the forces of attraction between the particles. (eg. bonds or intermolecular forces)
Changing state is a _______ change.
physical, as the particles and chemical properties of substance remain the same.
The change of state due to changes in temperature or pressure are called…
…interconversions of state.
What is the process of turning a substance from solid→gas called?
sublimination
Why are physical changes easy/hard to reverse?
easy, because no new substance is formed during interconversion of state.
Factors which affect evaporation…
larger surface area
higher temperature of liquid
Where does evaporation occur?
Only occurs at the surface of liquids where high energy particles can escape from the liquids surface at low temperatures, below the boiling point of the liquids
What happens during condensation?
When a gas is cooled at the particles collide, they do not have enough energy to bounce away so they make bonds and form a liquid.
Ionic Compounds Properties
conducts electricity, but not in the solid state
high melting/boiling points
Small Molecules (Covalent) Properties
low melting and boiling points
cannot conduct electricity
Giant Covalent Molecules Properties
high melting and boiling points
cannot conduct electricity (exception is graphite)
Examples of Giant Covalent Structures
Diamond
Graphite
Graphene
Fullerenes
Polymers
Diamond Structure
each carbon atom is bonded to 4 other carbon atoms by strong covalent bonds
the carbon atoms form a regular tetrahedral network structure
no free electrons (so doesn’t conduct electricity)
Diamond Properties and Uses
Useful for cutting tools due to its hard strucutre
Graphite Structure
each carbon atom is bonded to 3 other carbon atoms
carbon atoms form layers of hexagonal rings
no covalent bonds between layers, only intermolecular forces
one delocalised electron from each atom
Graphene structure
A single layer of graphite.
Fullerenes Structure
hexagonal rings of carbon atoms
joined by covalent bonds
some include rings with 5 or 7 carbon atoms
Graphite Uses
conducts electricity, so useful for electrodes for batteries and electrolysis
weak forces between layers means they can slide, so useful for pencils and lubricant
Graphene Properties and Uses
high melting point
very strong
delocalised electrons free to move across its surface, so conducts electricity
These properties make graphene useful for making composites.
Fullerenes Definition and Examples
These are molecules of carbon atoms with hollow shapes. Buckminsterfullerene and Nanotubes.
Composites Definition
Material made from 2 or more different materials with contrasting properties.
Buckminsterfullerene Description
1st fullerene to be discovered
made up of 60 carbon atoms (C) joined together by strong covalent bonds
spherical
weak intermolecular forces, so slippery and low melting point
Nanotube Structure , Properties and Uses
layer of graphene rolled into a cylinder
length is much longer than width, so have a high length:diametre ratio
high tensile strength - strong in tension and can resist being stretched
strong because of delocalised electrons
These properties make them useful in nanotechnology, electronics and specialised materials.
Tensile Strength Definition
The tension a material can withstand without breaking.
What is common between all polymers?
They’re all made up of smaller molecules called monomers.
Polymers - Boiling/Melting Ppoint explanation
Although they have strong covalent bonds, these are not what we need to break when changing state, but we need to break the intermolecular forces between the molecules. These require less energy to break, but because the polymers are so long with such a high surface area, there are many of these intermolecular forces, so it still requires quite a lot of energy to change their state.
Therefore, although they have lower melting points that giant covalent and giant ionic structures, they have higher melting points than simple molecular substances.
Why are metals conductors?
Electrical Conductors - delocalised electrons carry charge through the metal
Thermal Conductors - delocalised electrons transfer energy
High Melting/Boiling Points - metallic bonding is very strong so large amounts of energy are needed to overcome these bonds.
Alloy Definition
A mixture of at lease 2 or more elements, at least 1 of which is a metal.
Why can alloys be useful?
Many pure metals are too soft for many uses, so they’re made harder by adding another element, forming an alloy.
Why is an alloy harder than a pure metal?
Pure metals- giant metallic structure in solid state, and are arranged in layers which slide over each other when force is applied. In a pure metal, it requires little force for layers to slide.
Alloys - the atoms are of different sizes, so the smaller or bigger atoms distort the layers of atoms in the pure metal, meaning a greater force is required for the layers to sli9de over each other. This means it’s harder and stronger.
The greater the force needed, the harder and stronger the metal.
Nanoscience Definition
The study of structures that are between 1 and 100 nanometers in size.
Diameter of Atoms and Small Molecules
0.1nm
Diameter of Nanoparticles
1 - 100nm
Diameter of Fine Particles
100 - 2,500nm
Diameter of Coarse Particles
2,500 - 10,000 nm
Thickness of Paper
100,000 nm
Nanoparticles have very _____ surface area to volume ratios.
large
For a solid, the ________ its particles, the greater the surface area to volume ratio.
smaller
Surface Area to Volume ratio Equation
surface area (nm2) / volume (nm3)
A substance that consists of nanoparticles is described as ____________.
nanoparticulate
Uses of Nanoparticles
medical treatments
cosmetics, deodorants and sunscreens
electronics
catalysts
How are nanoparticles used in suscreens?
Sunscreens block harmful ultraviolet light from the sun reaching the skin. Zinc oxide blocks ultraviolet, so it is used in sunscreens, but it is used asd nanoparticles because it is white in bulk and invisible on skin as nanoparticles.
How are nanoparticles used as catalysts?
As they have a high surface area to volume ratio due to their small size, they are more efficient catalysts than larger catalyst molecules.
Self-cleaning window panes have nanoparticle coatings, acting as catalysts in the breakdown of dirth through the presence of sunlight.
Risks of Nanoparticles
There are concerns about them being breathed in and entering cells.
They could then catalyse harmful reactions in the body.
Toxic substances could bind to them because of their large SA:V, harming health.
Modern nanoparticle materials have only become common recently, so it is difficult to determine their risks.