Gr. 12 Chemistry: Quantum Mechanics
1/14
Earn XP
Description and Tags
atomic thoeries, electron configuration, quantum numbers
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
What are the four men involved in the atomic theories?
Dalton, Thomson, Rutherford, Bohr
Dalton:
solid billiard ball - no subatomic particles
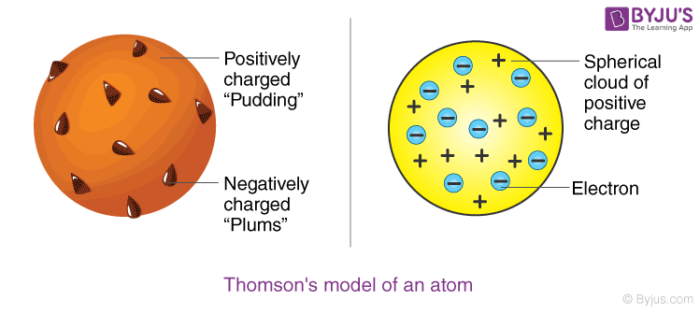
Thomson:
choc. chip muffin model - electrons like choc. chips embedded in positively charged atom
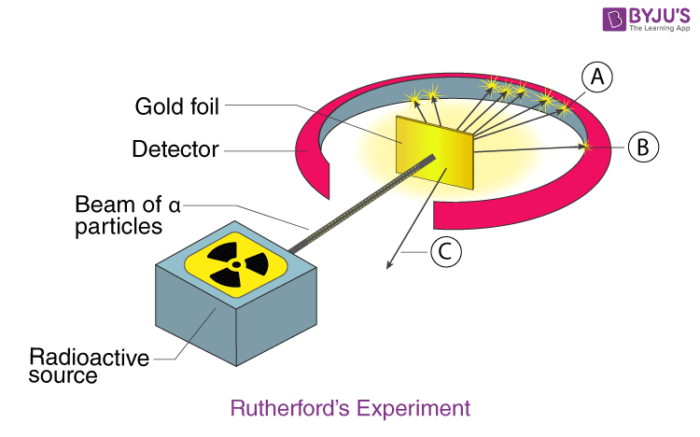
Rutherford:
nuclear model, +, tiny dense nucleus gold foil expt.
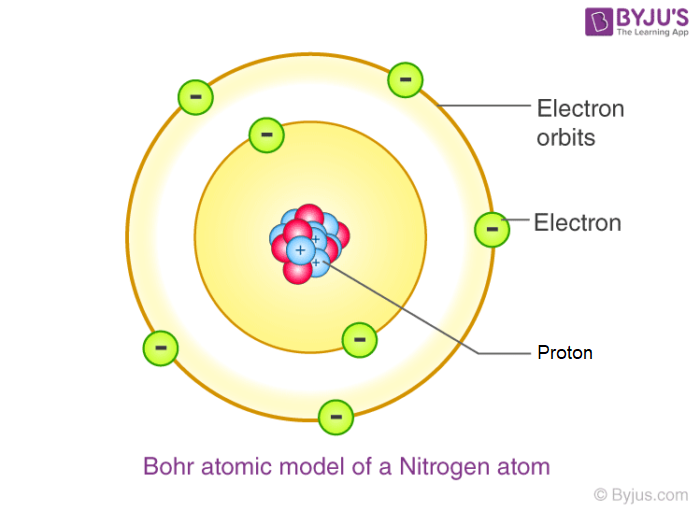
Bohr:
solar system model, electrons travel in orbits, fixed pathways around + nucleus
→ gives us the quantum number (n) principal quantum # energy level
ELECTRON CONFIGURATION:
how electrons are arranged in an atom’s shells and orbitals
AUFBAU PRINCIPAL:
lowest energy sub-level filled before moving on to the next sub-level (s<p<d<f)
PAULI EXCLUSION PRINCIPAL:
no two electrons can have the same 4 quantum numbers within an atom, they must have an opposite spin
HUND’S RULE:
spread out electrons in the same sub-level before doubling
QUANTUM NUMBERS
define an electron’s atomic orbital, including its energy level, shape, spatial orientation, intrinsic spin
The principal quantum #: