Renewables
1/128
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
129 Terms
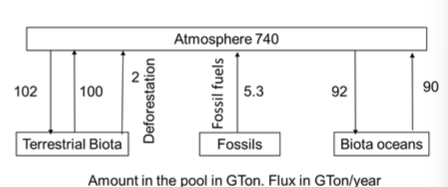
Assume that the flow from other pools into/out the atmosphere are negligible. What is the percent change in net carbon flux into the atmosphere if we cut emissions from fossil fuels by 10%?
16%
prime mover rotates at 3600 rpm exerting a torque of 30 Nm to provide mechanical power to an electrical generator. The mechanical power available from the prime mover is approximately ______
11 kW
Consider a 3-blade wind turbine with 10 m long blades. At a particular day and time, the wind speed at the height of the rotor is 10 m/s. Assume the air density is 1.225 kg/m3. Approximately, how much wind power is available for the turbine?
192 kW
An electrical load consumes 864 MJ during one day demanding constant power during this day. What is this energy in kWh and what is the power demand of this load during this day?
240 kWh and 10 kW
Consider a photon flux of one mmol/(s m2) at two different wavelengths 460 nm and 390 nm. The power flux at 390 nm is _________ the one at 460 nm.
1.18 times
A mass of 1000 kg molten salt with specific heat capacity 1.54 J/(gK) is used to store heat in a solar thermal facility (one type of renewable power production). How much heat is available to drive an engine for electricity production, if the salt reaches deltaT = 400K of temperature with respect to ambient temperature?
616MJ
A hydrogen fuel cell consumes 1 g/min of hydrogen. This mass rate is constant for 1 hour. Assume 60% efficiency. Approximately, what was the electrical power produced by this fuel cell during that hour?
1.43 kW
When burning one gram of methane the energy produced is approximately ___________ and the mass of CO2 produced is approximately ____________.
-50 kJ and 2.75 g
Why does the Mauna Loa CO2 data show an annual fluctuation?
Growing season of the northern hemisphere
Which option contains all greenhouse gases?
C02, CH4, H2O(g)
Long wave radiation
Outgoing
Short wave radiation
incoming
As a percentage, abundance of CO2 in the atmosphere is _____while nitrogen is ______
low, high
Most of the earth’s carbon is in the _____
sedimentary deposits and rocks
Assume productivity is 0.2kgm-2/d and biomass at day 30 is 2 kgm-2. What is the biomass in kgm-2 at day 31?
2.2
Biomass
kg/m2
primary product
plant
productivity
kg/(m2yr)
nutrients
cycle
secondary producer
herbivore
energy
flow
The term most appropriate for an energy conversion based on a resource that will last a very long time given the expected consumption rate is ____ and for an energy conversion based on a process that includes emission controls to avoid air pollution is ____.
renewable, clean
approximately what are the percentages of Carbon-based and non carbon based electricity production worldwide?
60 and 40
Compare the enthalpy of 2 chemical reactions. Enthalpy of reaction 1 is -200kJ/mol and reaction 2 is +250kJ/mol. We want to use the energy of one of these reactions to drive an engine. Which would yield more energy to drive the engine?
Reaction 1
What is the efficiency of a heat engine extracting 3MJ from the hot reservoir and delivering work of 1MJ?
0.33
Assume a closed thermodynamic system that absorbs 20 kJ of heat from the surroundings to increase its internal energy by 10 kJ. What is the correct staement regarding work?
System performed 10kJ work on surroundings
Inductor at steady state or DC
short circuit
capacitor at steady state or DC
open circuit
supercapacitors ____ than batteries
have lower energy density
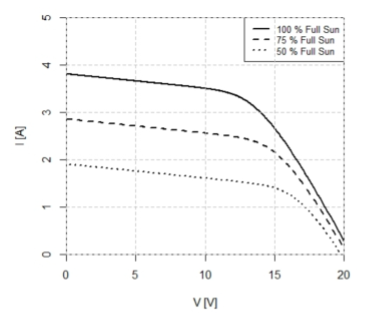
At 75% full sun, the power provided to the load when the output voltage is 15V is approximately
30W
Which one is the single most imprtant word to characterise module 2?
carbon
Order these compounds in terms of enhancing the greenhouse effect due to their radioactive properties. Go from high to low
CH4, CO2
methane hydrates are____ there are large deposits _____
clathrate…. the ocean floor
solar fuels are
a term to refer to making the equivalent fossil fuels in a hurry
natural gas
complex hydrocarbons
oil
hydrocarbon terms: large plants
coal
hydrocarbon term: algae and zooplankton
oil and gas
quaternary
holocene
paleozoic
carboniferous
mesozoic
jurassic
earth’s global surface____ data____ shows and increase that can be fit well by ___model.
5-year average of temperature anomaly with respect to 1951-1980…. since 1980…. a doubly exponential.
earth’s global surface temperature shows an increasing trend with respect to the _____ takens as a baseline reached approximately ____ in the early 2000s.
1951-1980 average…. 0.5C
Select the best combination to fill the blanks: the carbon in the CO2 emissionof coal-fired power plants can be sequestered before release at the stack by ____ that requires______ because of____.
absorption or adsorption column… regeneration,,,, saturation
ESP
uses electricity to remove fly ash particles by precipitation
SCB
use ammonia to remove NOx from te flue gas
FGD
uses a limestone slurry to remove sulfur before release of the flue gas at the stack
Rank from high to low thees three types of coal fire power plants in terms of efficiency and reduced CO2 emission: subcrit, SC, and USC
USC,SC, subcritical
sub-bituminous
intermediate carbon content and HV
anthracite
highest carbon content and HV
lignite
low carbon content and HV
bituminous
high carbon content and HV
Heat Rate
heat required to produce 1kWh
Heat Value
energy released by burning 1kg of coal
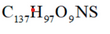
what is the carbon content in percent of coal with formula:
85
Which one is not part of the characteristics to describe a type of coal?
algae content
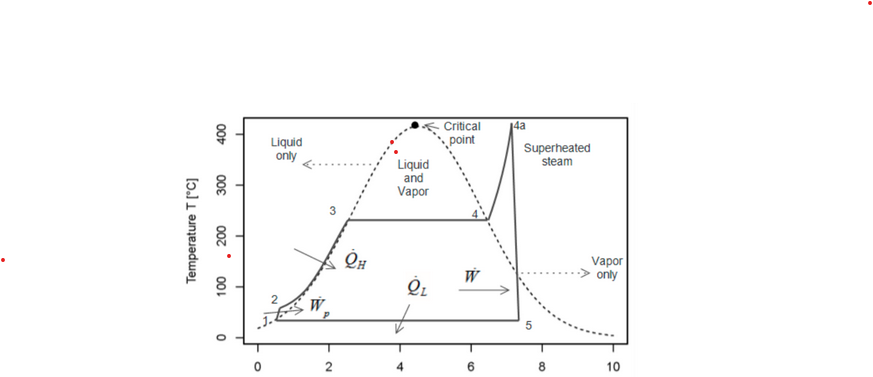
The path from state 2 to state 3 is approximately ____ with water in the ____phase, whereas the path from 4a to 5 is approximately ___ with water____ phase
isobaric, liquid, isentropic, changing
Peaking Power plant
runs when needed to cover demand
baseload powerplant
runs continuously
which choice best matches power plants that use rankine cycle?
coal-fired. geothermal, nuclear
Energy term: electricity
a fraction of total energy prodcution
Energy term: toe
energy obtained by burning 1 ton of oil
TPES
annual world energy consumption
EJ
convenient unit for energy reserves
The reactants for a hydrogen fuel cell are ____ and the byproducts are____
hydrogen and oxygen…. water and heat
the difference between a battery and a fuel cell is___
that a fuel cell requires constant inflow of reactants whereas the battery does not
A fuel cell converts ____ directly to ____
chemical energy…. electricity
Gibb’s free energy G is _____ minus____
enthalpy….heat
An isobaric reversible process experiments an increase of enthalpy 100kJ. The heat added was
100kJ.
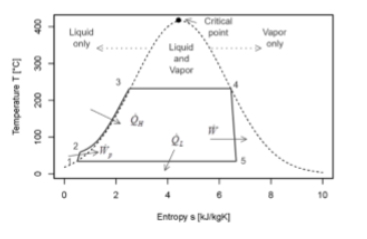
Consider the thermodynamic cycle traced by the solid lines. This is a _____ cycle and the 4-5 path at the right hand side is nearly____
rankine and isentropic.
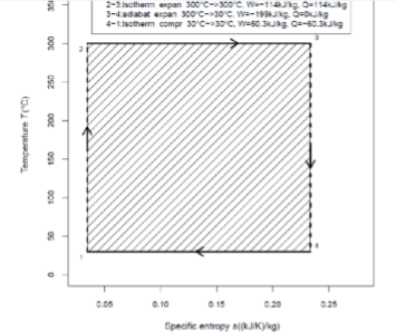
This is ____ cycle and the path from state 4 to 1 is____
a carnot; a reversible isothermal compression
An isothermal reversible process at 300K experiments an increase of entropy equal to 1kJ/K. What is the heat Q transferred"?
300kJ

The path from 1 to 2 is an ____ and requires____ than the path from 3 to 4 which is an ____.
isothermal expansion; less heat; isothermal expansion
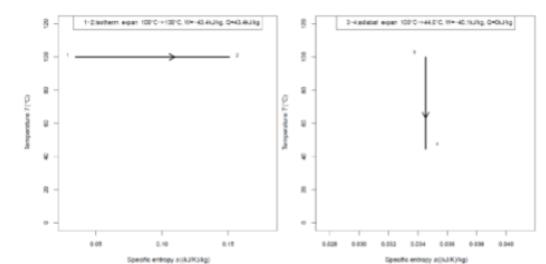
The path from 1 to 2 is an ____ with ____ whereas the path from3 to 4 is an____ with ____.
Isothermal with increasing entropy; adiabat with constant entropy
For irreversible thermodynamic processes entropy always ____, whereas for reversible processes entropy____.
increase…. remains constant
the PF for an inductor is ___, for a capacitor is____, and for a resistor is _____.
0….0….1
The duty cycle for a ____ DC-DC converter to from 10V to 30V is ____.
boost… 0.666
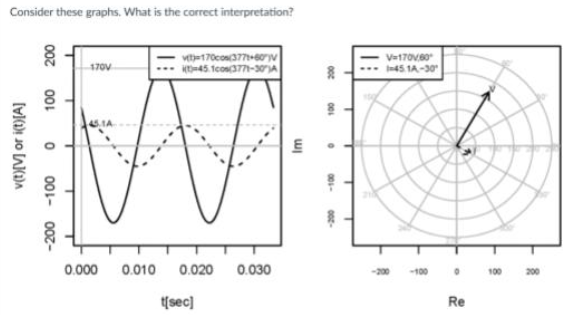
Consider these graphs what is the correct interpretation?
corresponds to aa 10 mH inductor
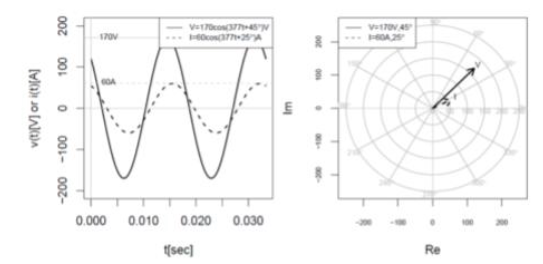
Which statement about the voltage is correct?
The current lags the voltage by 20
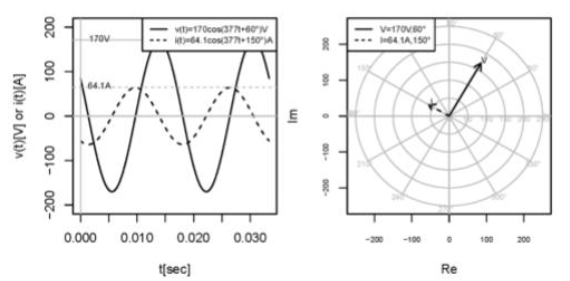
What is the correct interpretation of the graph?
corresponds to a 1mF capacitor
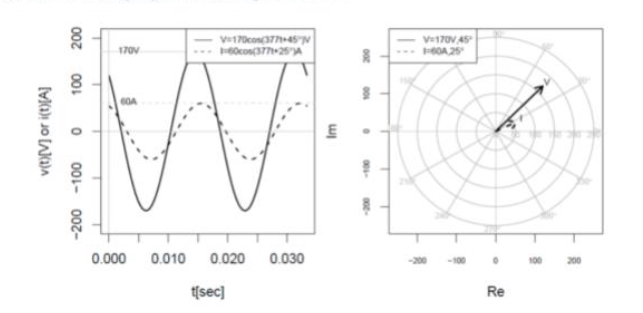
What does this graph correspond to?
A resistor of 2 ohms
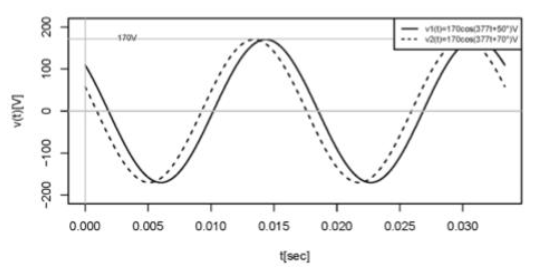
What is the correct phase difference interpretation
V1 lags V2 by 20
A rotor with only one magnet (only one S-N pole pair) must rotate at ___ for voltage to be____.
3600 rpm… 60Hz
in a generator the rotating____ produces a sinusoidal variation of ____ which by Faraday’s law becomes a sinusoidal____.
magnet…. magnetic flux… voltage
steam turbine
one type of prime mover
prime mover
generic term of machine that spins a generator
motor
output torque input electrical power
generator
input torque produces electrical power
An OTEC power plant does not vaporise water to produce steam because
the warm surface ocean water is not sufficiently hot
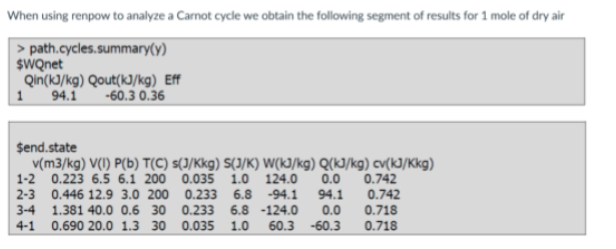
the system has heat source at ____ heat sink at ___ and the work is _____
200C, 30C, 33.8kJ/kg
For an ideal Carnot cycle: the isothermal expnasion from volume V2 to volume V3 yields____ the isothermal compression from volume V4 to volume V1
same ratio as
Find the best set of power generation plants that employ a Rankine thermodynamic cycle
coal-fired, geothermal, nuclear
turbine
spins by the steam
boiler
add heat to produce steam
generator
moved by the turbine
steam power plant
rankine cycle
condenser
steam to liquid water
high QL/QH
low efficiency
low QL/QH
high efficiency
geothermal electricity production ____ but is ___ everywhere in the world
can be considered renewable…. not available