chemistry - condensation polymers
1/9
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
10 Terms
what polymers are formed by condensation polymerisation?
polyesters
what is a by product of condensation polymerisation?
water
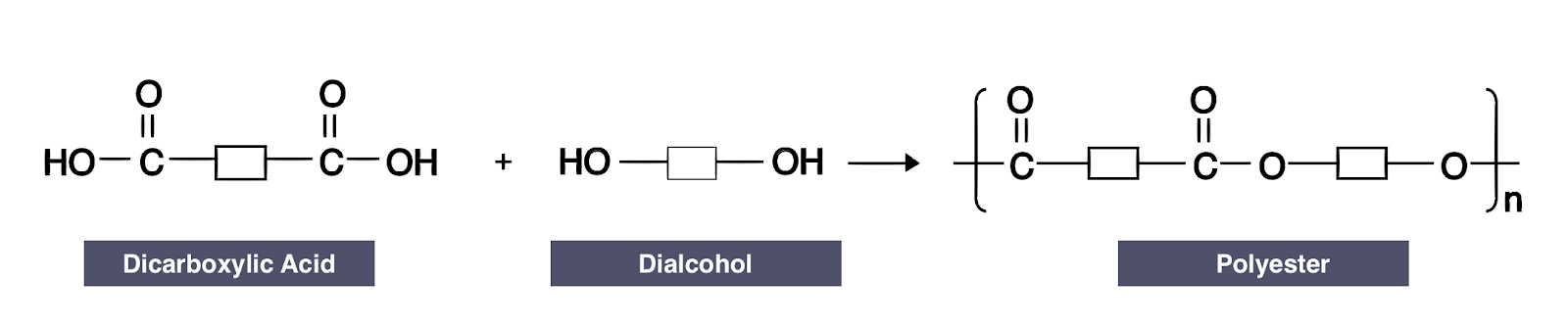
what monomers are used to make polyesters?
a diol and a dicarboxylic acid
what is a diol?
an alcohol with 2 OH groups, one at each end
what is condensation polymerisation?
formation of long chain molecules by lots of small monomers joining together, each with two functional groups which react with each other
what is a dicarboxylic acid?
a carboxylic acid with two COOH groups, one at each end
give the reaction for condensation polymerisation
diol + dicarboxylic acid → polyester + water
how do you draw the repeat unit of a polyester?
remove the 2 OHs from the dicarboxylic acid
remove the end Hs from the diol
join the two together, bracket, and n h
are any polyesters biodegradable?
yes
what are biodegradable polyesters called?
biopolyesters - means they can decompose, don’t stay in landfill, and are less polluting