Accumulations
1/12
Earn XP
Description and Tags
Proteins, Lipids, Glycogen, etc
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
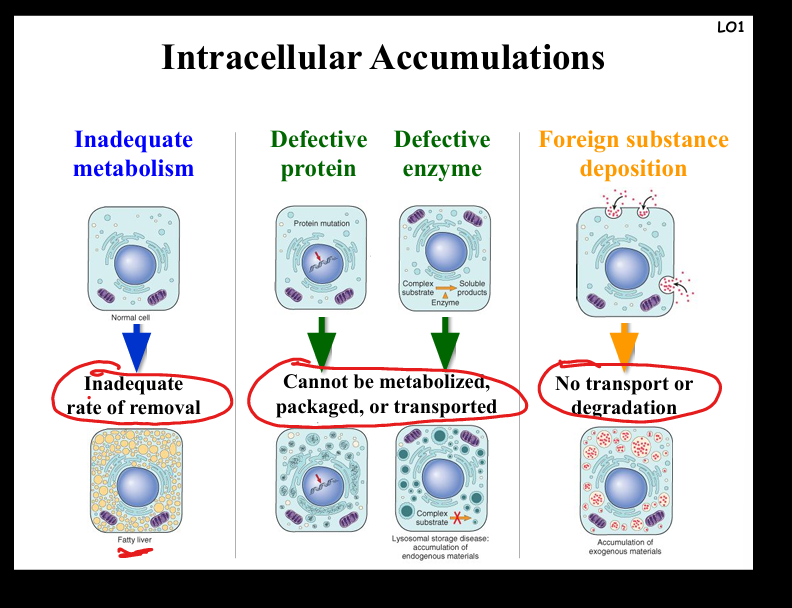
Describe the causes and pathogenesis of intracellular accumulations
Mechanisms: (1) Inadequate removal of normal substances (e.g. steatosis in fatty liver from alcohol, diabetes, obesity); (2) Accumulation of abnormal endogenous substances due to folding/transport defects (e.g. α1-antitrypsin deficiency → cirrhosis, emphysema); (3) Failure to degrade metabolites due to enzyme deficiencies (e.g. glycogen storage diseases, Pompe disease); (4) Deposition of abnormal exogenous substances (e.g. carbon particles in anthracosis). Clinical examples: fatty liver, atherosclerosis, neurodegenerative proteinopathies, lysosomal storage diseases.

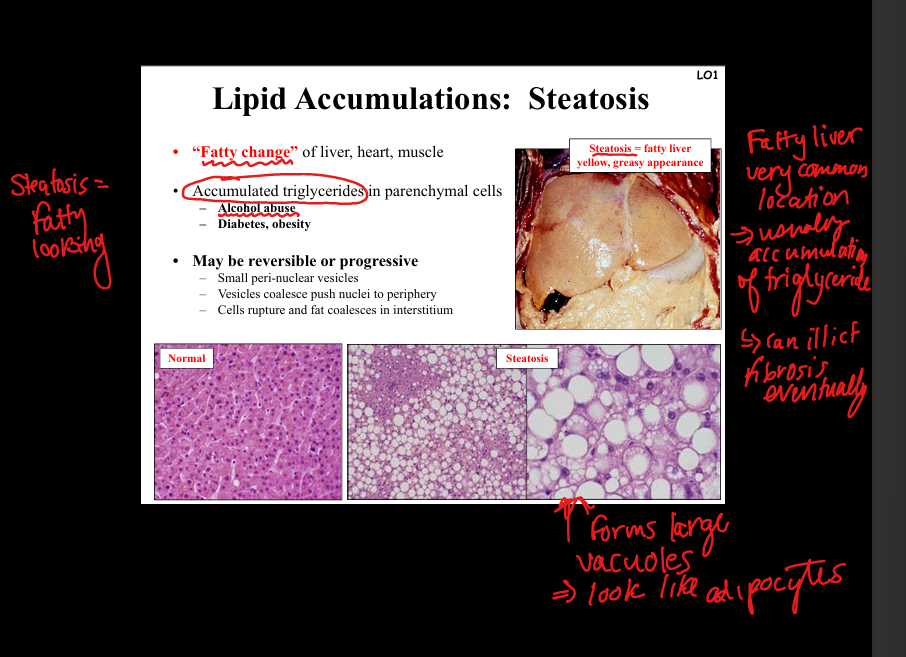
Describe steatosis (fatty change).
Accumulation of triglycerides in parenchymal cells (liver, heart, muscle). Causes: alcohol abuse, diabetes, obesity. Pathology: small perinuclear vesicles coalesce, push nucleus to periphery; cells rupture → fat in interstitium. Gross: yellow, greasy liver. Clinical: reversible but may progress to fibrosis/cirrhosis.

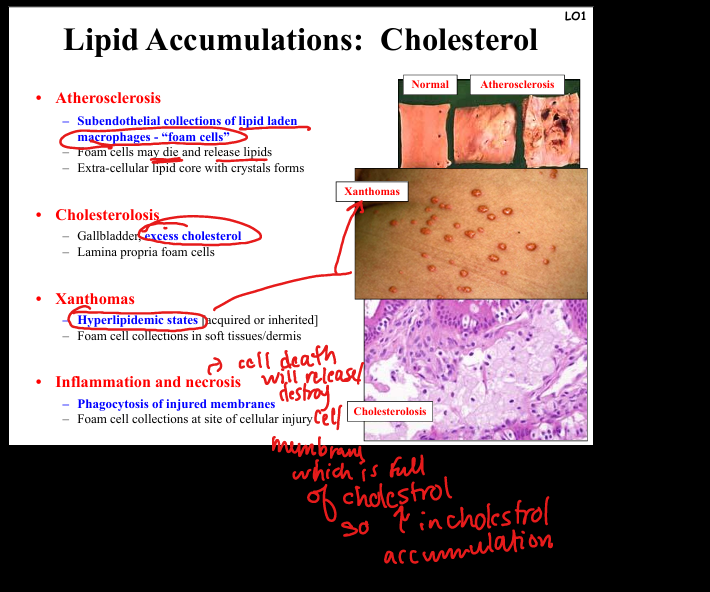
Describe cholesterol accumulation.
Seen in atherosclerosis (foam cells in intima, lipid core with crystals), cholesterolosis (gallbladder lamina propria foam cells), xanthomas (foam cell collections in soft tissue/dermis in hyperlipidemic states). Pathogenesis: macrophages engulf cholesterol, die, release lipids → extracellular deposits.

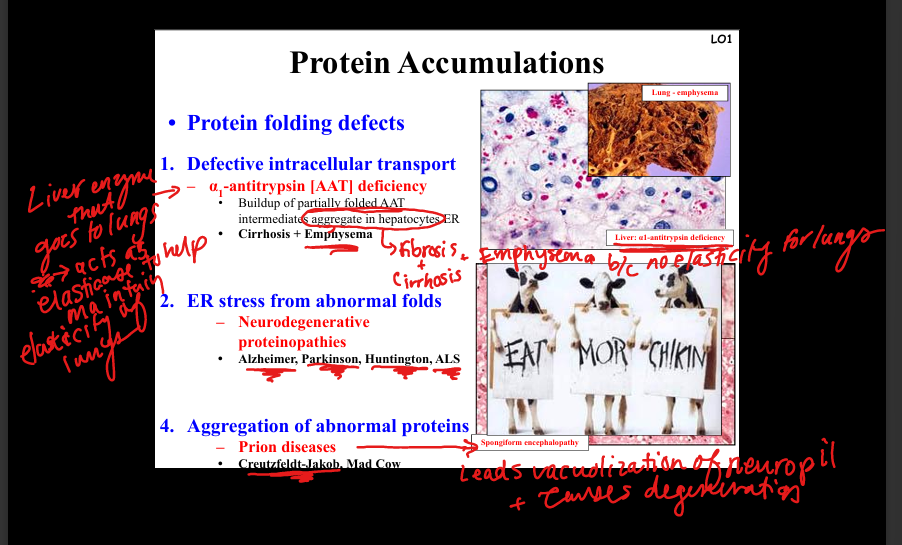
What is α1-antitrypsin deficiency?
Defective intracellular transport of misfolded α1-antitrypsin in hepatocyte ER → aggregates → cirrhosis. Lack of functional AAT in lungs → loss of elastase inhibition → emphysema.

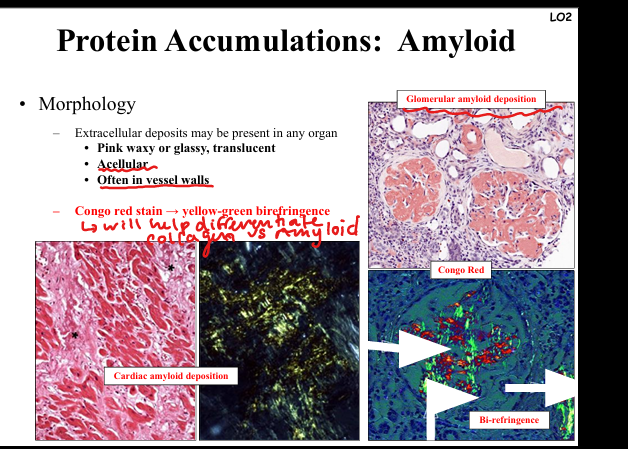
How is amyloid diagnosed morphologically?
Extracellular pink, waxy, glassy deposits in any organ, often vessel walls. Congo red stain → apple-green birefringence under polarized light. Seen in kidney glomeruli, myocardium, GI tract.

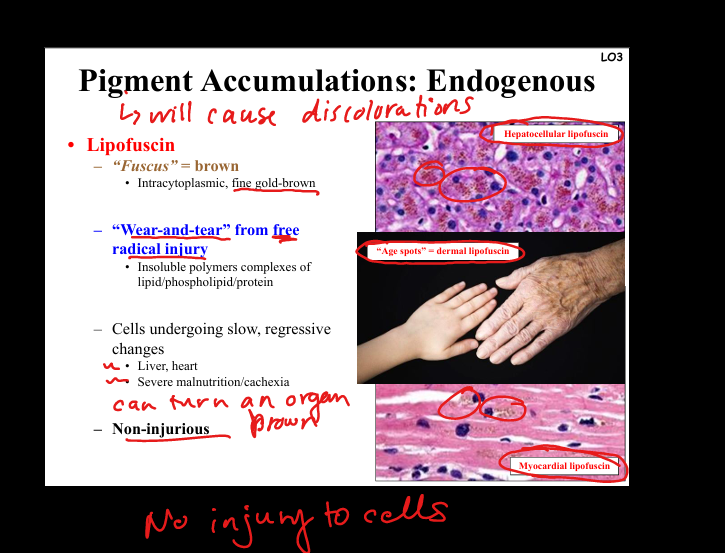
Describe lipofuscin.
“Wear-and-tear” pigment. Golden-brown, intracytoplasmic granules from lipid peroxidation/free radical injury. Seen in aging heart, liver, cachexia. Non-injurious marker of oxidative stress.

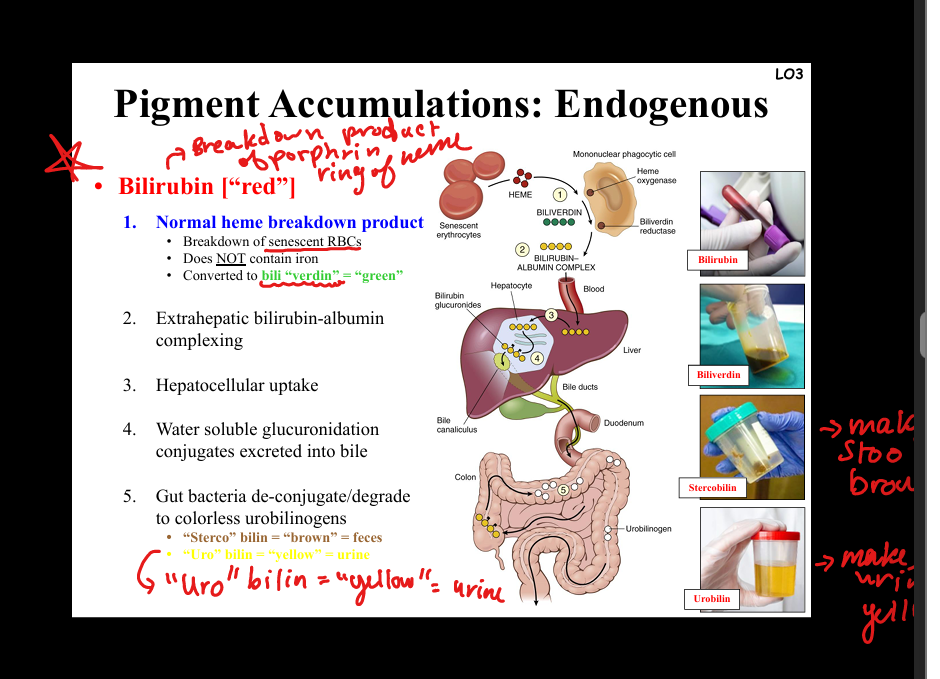
Describe bilirubin accumulation.
Heme breakdown product (does not contain iron). Normal metabolism: heme → biliverdin → bilirubin → conjugation in liver → excretion in bile → gut bacteria → stercobilin (brown feces), urobilin (yellow urine). Pathology: excess bilirubin → jaundice (scleral icterus, kernicterus in brain). Causes: hemolysis, liver dysfunction, biliary obstruction.

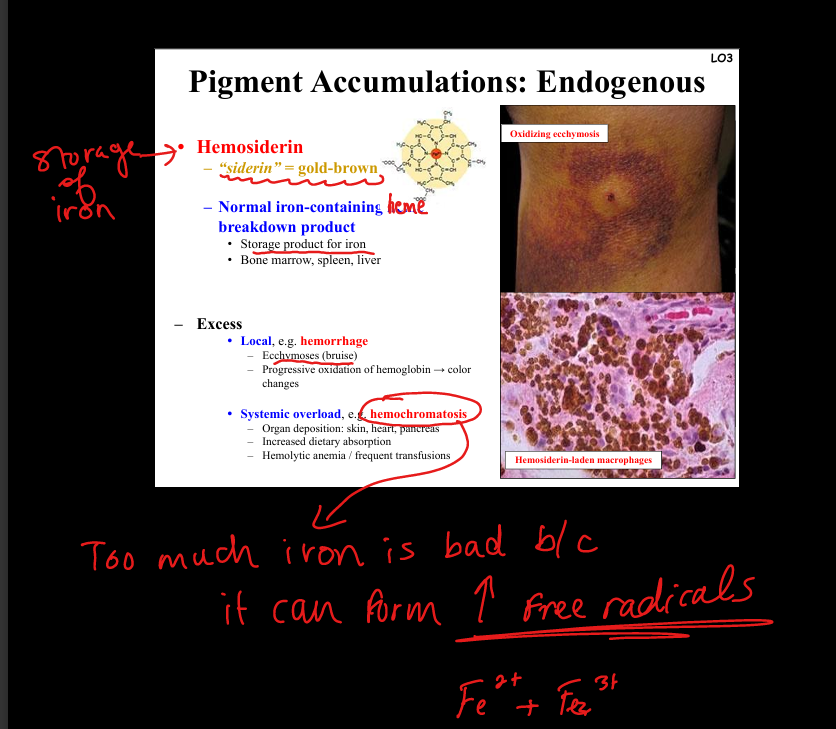
Describe hemosiderin accumulation.
Iron storage pigment, golden-brown. Local excess: hemorrhage → ecchymosis (bruise, color changes). Systemic overload: hemochromatosis (iron deposition in skin, heart, pancreas). Pathogenesis: excess iron → free radical formation → organ damage.

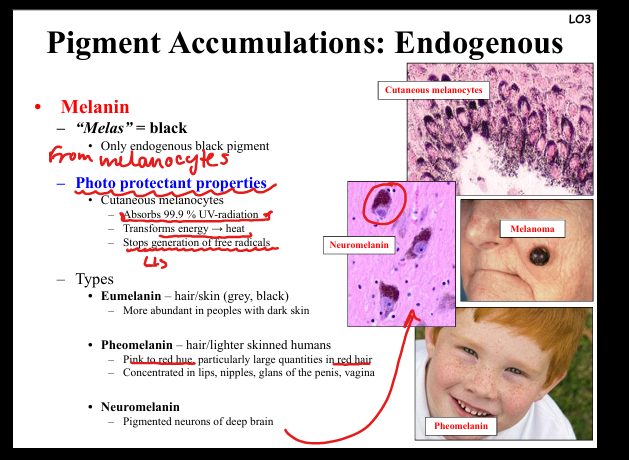
Describe melanin accumulation.
Black pigment from melanocytes. Functions: absorbs UV radiation, prevents free radical formation. Types: eumelanin (dark skin/hair), pheomelanin (red hair, lips, nipples, genitalia), neuromelanin (pigmented neurons). Pathology: melanoma (malignant proliferation).

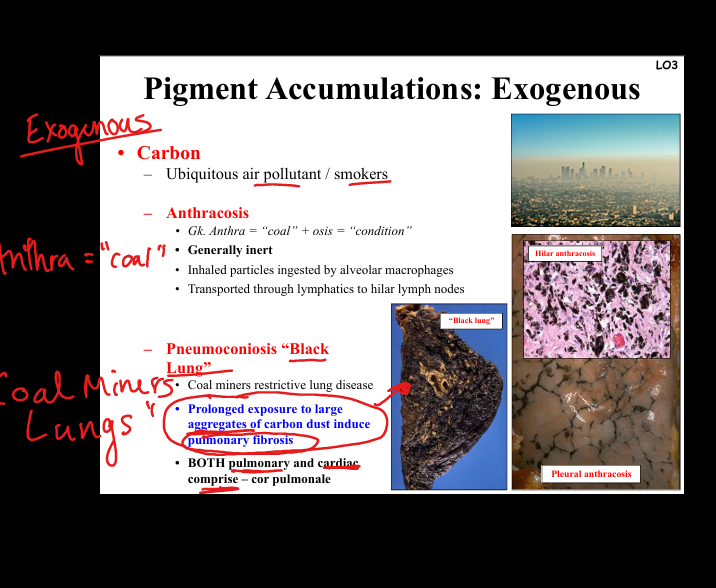
Describe carbon accumulation.
Exogenous pigment. Anthracosis = inert carbon particles in lung macrophages, transported to lymph nodes. Pneumoconiosis (“black lung”) = coal dust exposure → pulmonary fibrosis, restrictive lung disease, cor pulmonale. Seen in coal miners, smokers.

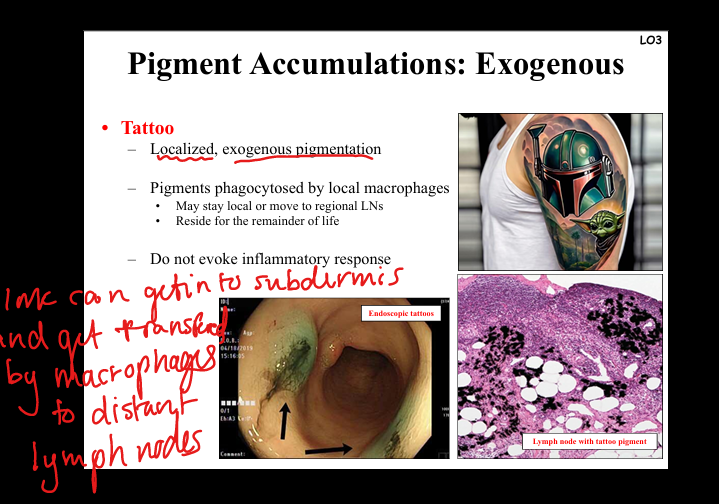
Describe tattoo pigment accumulation.
Exogenous pigment phagocytosed by dermal macrophages, may migrate to lymph nodes. Usually inert, persists lifelong, no inflammatory response.

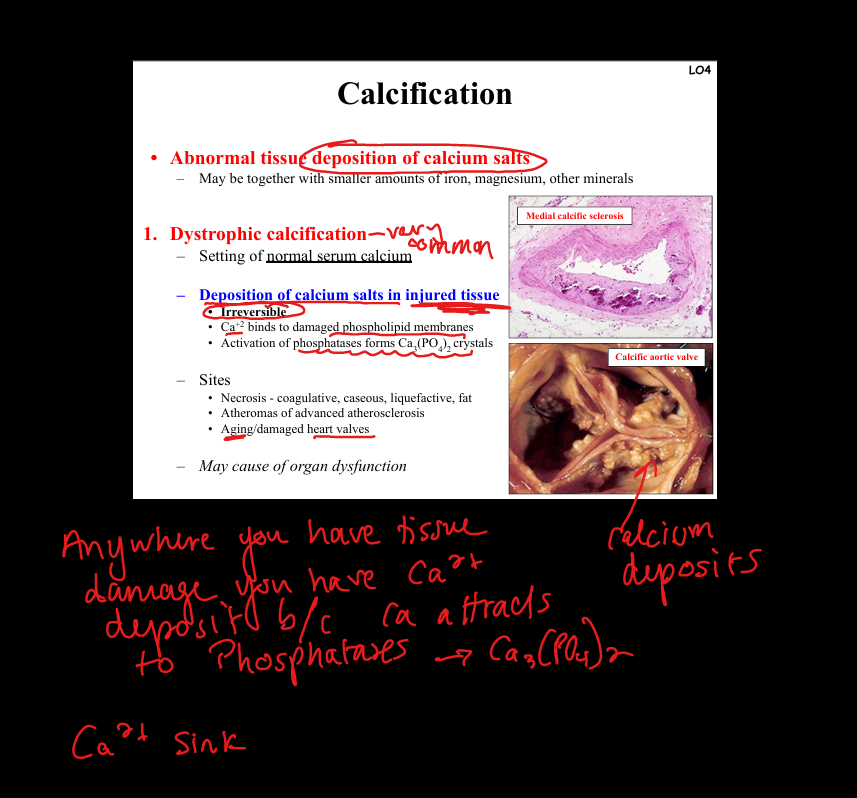
Define dystrophic calcification.
Deposition of calcium salts in injured/necrotic tissue despite normal serum calcium. Pathogenesis: Ca²⁺ binds damaged membranes → CaPO₄ crystals. Sites: necrosis (coagulative, caseous, fat), atherosclerotic plaques, damaged heart valves. Clinical: may impair organ function (e.g. calcific aortic stenosis).

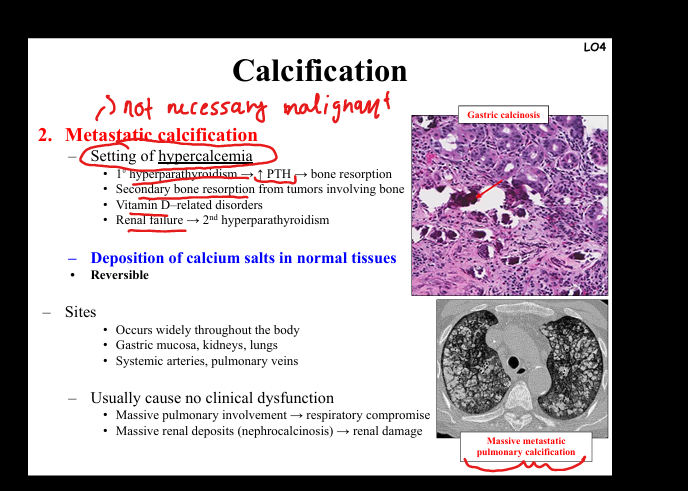
Define metastatic calcification.
Deposition of calcium salts in normal tissues due to hypercalcemia. Causes: primary hyperparathyroidism, bone destruction from tumors, vitamin D disorders, renal failure (secondary hyperparathyroidism). Sites: gastric mucosa, kidneys, lungs, systemic arteries. Clinical: widespread deposits, may cause respiratory compromise or nephrocalcinosis.