Buffers and Henderson-Hasselbalch Eq
0.0(0)
Card Sorting
1/31
Last updated 10:41 AM on 12/12/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
1
New cards
Buffers
They are solutions that resist changes in pH when an acid or base is added
2
New cards
How do buffers act
They act by neutralizing acid or base that is added to the buffered solution
3
New cards
Composition of Buffers
Buffers are made by mixing a solution of a weak acid with a solution of soluble salt containing its conjugate base anion
- weak acid + conjugate base salt
- weak acid + conjugate base salt
4
New cards
Buffer composition of blood
Mixture of H2CO3 (Carbonic Acid) and HCO3- (Bicarbonate)
5
New cards
How Acid Buffers work: Addition of Base
If a strong base is added to a buffer, the weak acid will give up its H+ in order to transform the base (OH-) into water (H2O) and the conjugate base
HA + OH- → A- + H2O.
HA + OH- → A- + H2O.
6
New cards
What happens if a strong base is added to a buffer solution
The amount of the weak acid decreases while the amount of the conjugate base increases
7
New cards
How Acid Buffers work: Addition of Acid
If a strong acid is added to a buffer, the weak base will react with the H+ from the strong acid to form the weak acid
HA: H+ + A- → HA
- The H+ gets absorbed by the A- instead of reacting with water to form H3O+ (H+), so the pH changes only slightly.
HA: H+ + A- → HA
- The H+ gets absorbed by the A- instead of reacting with water to form H3O+ (H+), so the pH changes only slightly.
8
New cards
Common Ion Effect
HA(aq) + H2O → A-(aq) + H3O+ (aq)
Adding a salt containing the anion HA (conjugate base of the acid- the common ion), shifts the equilibrium to the left
Adding a salt containing the anion HA (conjugate base of the acid- the common ion), shifts the equilibrium to the left
9
New cards
What happens because of the Common Ion Effect
It causes the pH to be higher than the pH of the acid solution
- lowers the H3O+ ion concentration
- lowers the H3O+ ion concentration
10
New cards
Use of Henderson-Hasselbalch Equation
It calculates the pH of a buffer from the pKa and initial concentrations of the weak acid and salt of conjugate base
(as long as x is small, approxiation is valid)
(as long as x is small, approxiation is valid)
11
New cards
Henderson-Hasselbalch Equation
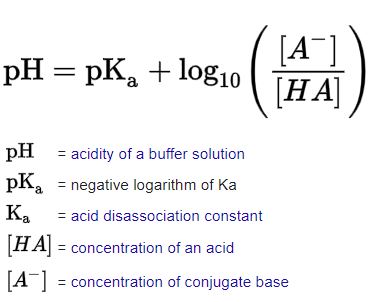
12
New cards
When to use the Henderson-Hasselbalch Equation
When the "x is small" approximation is applicable
13
New cards
When will the "x is small" approximation work
Both should be true
- The initial concentrations of acid and salt are not very dilute (should be over 100 to 1000x larger than Ka value)
- Ka is fairly small
- The initial concentrations of acid and salt are not very dilute (should be over 100 to 1000x larger than Ka value)
- Ka is fairly small
14
New cards
Basic Buffers
B:(aq) + H2O(l) → H:B+ (aq) + OH-(aq)
Buffers made by mixing a weak base (B:) with a soluble salt of its conjugate acid (H:B+Cl-)
Buffers made by mixing a weak base (B:) with a soluble salt of its conjugate acid (H:B+Cl-)
15
New cards
Henderson-Hasselbalch Equation for Basic Buffers
pH is baliktad, its log ([B:]/[H:B+])
![pH is baliktad, its log ([B:]/[H:B+])](https://knowt-user-attachments.s3.amazonaws.com/504725d1ffad4c9b96446b24a058f967.jpeg)
16
New cards
Buffering Capacity
amount of acid or base a buffer can neutralize
17
New cards
Buffering Range
pH range the buffer can be effective
18
New cards
Effectiveness of Buffers depends on
1. Relative amounts of acid and base
2. Absolute concentrations of acid and base
2. Absolute concentrations of acid and base
19
New cards
Conditions where buffers are most effective
1. MOST EFFECTIVE: equal concentrations of acid and base
2. EFFECTIVE: 0.1 < [base]:[acid] < 10
3. MOST EFFECTIVE: [acid] and [base] are large
2. EFFECTIVE: 0.1 < [base]:[acid] < 10
3. MOST EFFECTIVE: [acid] and [base] are large
20
New cards
Calculation of pH range (highest and lowest ph)
- highest: pKa +1
- lowest: pKa -1
- lowest: pKa -1
21
New cards
When choosing an acid to make a buffer, choose ...
Choose an acid whose pKa is closest to the pH of the butter
22
New cards
how to find the solution that has the highest buffer capacity
one that has the most components available to react with the added base/acid to resist change, highest concentration or M
23
New cards
Weak bases
NH4OH, N(CH3)3, C5H5N, NH4OH, H2O, C6H5NH2, NH3, PH3
24
New cards
Weak Acids
HClO2, HF, HNO2, HC2H3O2, HClO, HBrO, HCN, HIO, CH3COOH, H2S, H2CO3
25
New cards
Buffer solutions must contain
1. Relatively large concentration of acid to react with any OH ions to it
2. A similar concentration of base to react with any added H+ ions
2. A similar concentration of base to react with any added H+ ions
26
New cards
What should not happen during the neutralization reaction
Buffer's acid and base components must not consume each other
27
New cards
If the ratio of \[HA\]=\[A-\] then
pH = pKa
28
New cards
If \[HA\] > \[A-\] then
pH < pKa
29
New cards
If \[A-\] > \[HA\]
pH > pKa
30
New cards
What phenomenon accounts the mechanism of buffers
Common Ion Effect
31
New cards
What is Common Ion Effect
happens when an ion is added to a mixture (already in equilibrium) that already contains the same ion causes the position of the equilibrium to shift away from forming more of it.
32
New cards
Buffer capacity depends on what
amount of acid and base used to prepare a buffer
* the larger the amount of acid and base, the greater its buffer capacity
* the larger the amount of acid and base, the greater its buffer capacity