UNIT 1: Biological Molecules
1/118
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
119 Terms
What are monomers?
Monomers are the smaller units from which larger molecules are made.
What are polymers?
Polymers are molecules made from a large number of monomers joined together.
Give three examples of monomers.
Three examples of monomers are monosaccharides, amino acids and nucleotides.
What is a condensation reaction?
A condensation reaction joins two molecules together with the formation of a chemical bond and involves the elimination of a molecule of water.
What is a hydrolysis reaction?
A hydrolysis reaction breaks a chemical bond between two molecules and involves the use of a water molecule.
What are monosaccharides?
Monosaccharides are the monomers from which larger carbohydrates are made.
Give three examples of monosaccharides.
Three examples of monosaccharides are glucose, galactose and fructose.
Which bond is formed during the condensation reaction between two monosaccharides?
A condensation reaction between two monosaccharides forms a glycosidic bond.
How are disaccharides formed?
Disaccharides are formed by the condensation of two monosaccharides.
How is maltose formed?
Maltose is a disaccharide formed by condensation of two glucose molecules.
How is sucrose formed?
Sucrose is a disaccharide formed by condensation of a glucose molecule and a fructose molecule.
How is lactose formed?
Lactose is a disaccharide formed by condensation of a glucose molecule and a galactose molecule.
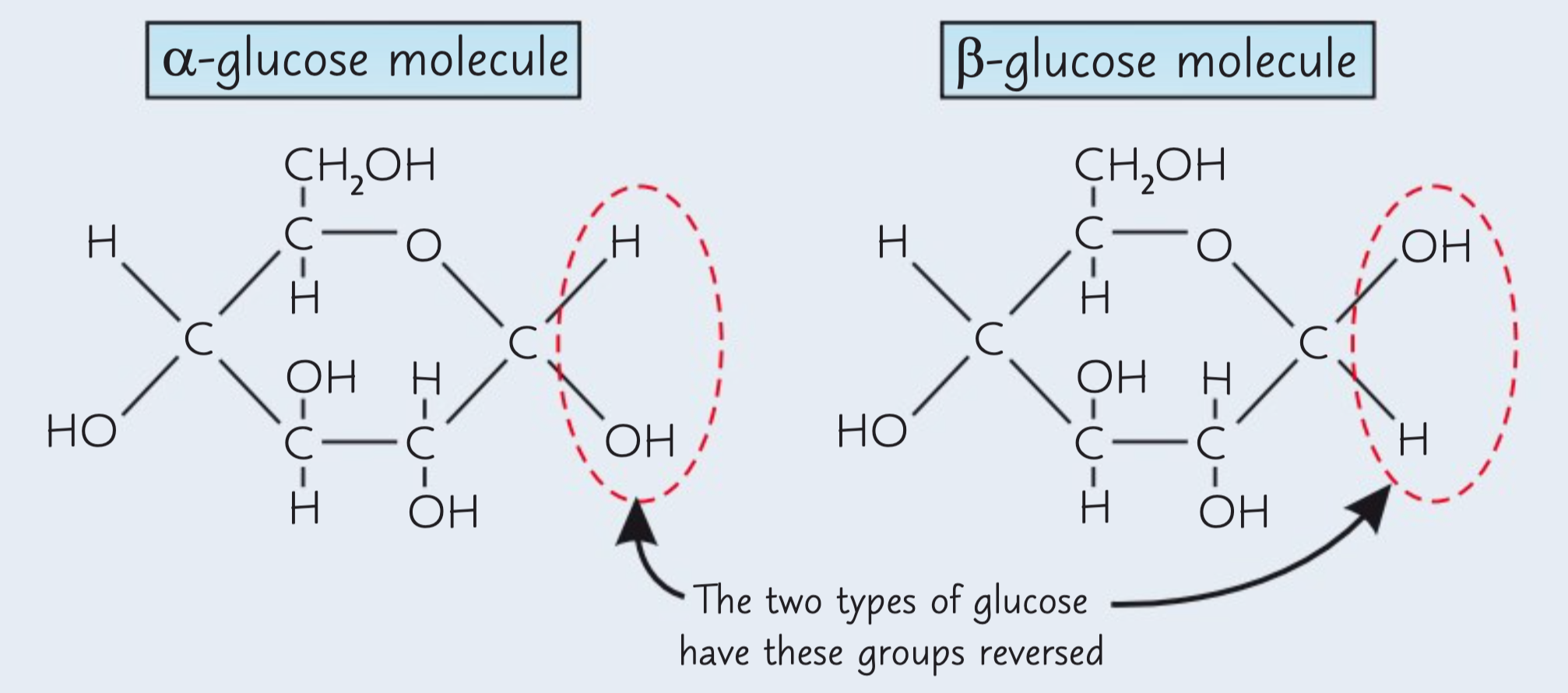
What are the two isomers of glucose?
Glucose has two isomers, α-glucose and β-glucose.
What are the structures of α-glucose and β-glucose?
How are polysaccharides formed?
Polysaccharides are formed by the condensation of many glucose units.
How are glycogen and starch formed?
Glycogen and starch are formed by the condensation of α-glucose.
What is the structure of glycogen?
Glycogen is a polysaccharide of α-glucose with C1-C4 and C1-C6 glycosidic bonds so it is branched.
Give three ways the structure of glycogen is related to its function.
Glycogen is branched so it can be rapidly hydrolysed to release glucose for respiration.
Glycogen is a large polysaccharide molecule which cannot leave the cell.
Glycogen is insoluble in water, so the water potential is not affected.
What is the structure of starch?
Starch is a mixture of amylose and amylopectin; amylose has C1-C4 glycosidic bonds so it is unbranched, while amylopectin has C1-C4 and C1-C6 glycosidic bonds so it is branched.
Give three ways the structure of starch is related to its function.
Starch is insoluble in water, so the water potential is not affected.
The angles of the glycosidic bonds in amylose give it a coiled structure - compact.
The side branches in amylopectin allow enzymes to reach the glycosidic bonds easily.
How is cellulose formed?
Cellulose is formed by the condensation of β-glucose.
Describe how structure of cellulose is related to its function.
Cellulose is made of long and straight unbranched chains.
These chains are linked with many hydrogen bonds to form strong fibres called microfibrils.
Describe the test for reducing sugars.
(monosaccharides + some disaccharides)
1. Add benedict's reagent (blue) to sample
2. Heat in a boiling water bath
3. Positive = green / yellow / orange / red precipitate (reducing sugar present)
Describe the test for non-reducing sugars.
1. Add a few drops of dilute hydrochloric acid
2. Heat in a boiling water bath
3. Neutralise with sodium hydrogen carbonate
4. Add Benedict's reagent and heat again
5. Positive = green / yellow / orange / red precipitate
Describe the test for starch.
1. Add iodine dissolved in potassium iodide to solution and shake/stir
2. Brown to Blue-black colour = starch present
What are the two groups of lipids?
The two groups of lipids are triglycerides and phospholipids.
How are triglycerides formed?
Triglycerides are formed by the condensation of one molecule of glycerol and three molecules of fatty acid.
Which condensation reaction forms an ester bond?
The condensation reaction between glycerol and a fatty acid (RCOOH) forms an ester bond.
Give two ways the structure of triglycerides is related to their function.
Triglycerides have long hydrocarbon tails, which contain lots of chemical energy. They can store lots of energy per gram.
Triglycerides are insoluble, so they don't affect the water potential of the cell.
Explain why triglycerides clump together in cells.
Describe how triglycerides clump together in cells.
The triglycerides clump together as insoluble droplets because the fatty acid tails are hydrophobic.
The tails face inwards, shielding themselves from water with their glycerol heads.
How are phospholipids formed?
In phospholipids, one of the fatty acids of a triglyceride is substituted by a phosphate-containing group.
Hence, they are formed by the condensation of a phosphate group, glycerol, and 2 fatty acids.
Explain why phospholipids form a bi-layer in water.
Phospholipids form a bi-layer in water because they hydrophilic heads and their tails are hydrophobic.
The heads face out towards the water on either side.
Why can't water soluble substances pass through phospholipids?
Water-soluble substances can't easily pass through the centre of the bilayer, as it is hydrophobic.
What are the two types of fatty acids?
How are they different?
Fatty acids may have a saturated or unsaturated R-group.
The unsaturated R-group will have one or more C=C double bonds.
Describe the test for lipids.
1. Add ethanol and shake (dissolves lipids)
2. Then pour into water
3. Positive: milky white emulsion
What are amino acids?
Amino acids are the monomers from which proteins are made.
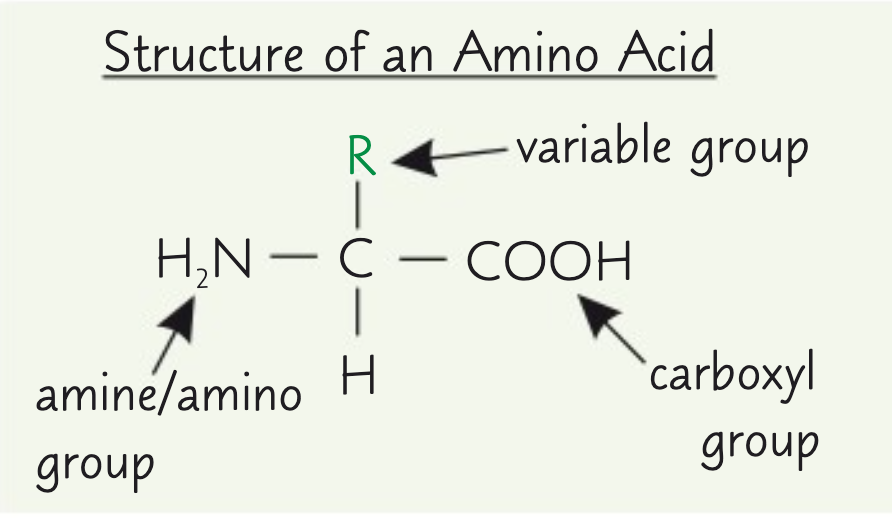
What is the general structure of an amino acid?
Where NH2 represents an amine group.
COOH represents a carboxyl group.
R represents a side chain.
How do the twenty amino acids differ?
The twenty amino acids that are common in all organisms differ only in their side group.
How are peptide bonds formed?
Peptide bonds are formed during the condensation reaction between two amino acids.
How are dipeptides formed?
Dipeptides are formed by the condensation of two amino acids.
How are polypeptides formed?
Polypeptides are formed by the condensation of many amino acids.
How many polypeptides may a functional protein contain?
A functional protein may contain one or more polypeptide.
Describe the primary structure of a protein.
The primary structure is the sequence of amino acids in the polypeptide chain.
Describe the secondary structure of a protein.
The secondary structure involves the hydrogen bonds formed between the amino acids in the chain.
This makes it coil into an alpha helix or fold into a beta pleated sheet.
Describe the tertiary structure of a protein.
The tertiary structure involves ionic bonds and disulfide bridges, as well as more hydrogen bonds between the R groups of the amino acids.
Describe the quaternary structure of a protein.
The quaternary structure involves the interactions and bonds between different polypeptide chains.
True or False.
The quaternary structure is always the protein's final 3D structure.
False.
For proteins made from a single polypeptide chain, the tertiary structure is their final 3D structure.
For proteins made from more than one polypeptide chain, the quaternary structure is their final 3D structure.
Describe the structure of an enzyme.
Enzymes are spherical in shape due to the tight folding of the polypeptide chains.
Describe the structure of antibodies.
Antibodies are made up of two light polypeptide chains and two heavy polypeptide chains bonded together.
In the variable regions, the amino acid sequences vary greatly.
Describe the structure of channel proteins.
Channel proteins contain hydrophobic and hydrophillic amino acids.
This causes the protein to fold up and form a channel.
Describe the structure of structural proteins.
Structural proteins have long polypeptide chains lying parallel to each other.
They have cross-links between them.
Describe the biuret test for proteins.
Add Biuret reagent. blue to purple
What does each enzyme do?
Each enzyme lowers the activation energy of the reaction it catalyses. The enzyme remains unchanged.
True or False.
Enzymes only catalyse intracellular reactions.
False.
Enzymes catalyse a wide range of intracellular and extracellular reactions.
True or False:
Enzymes affect structures and functions in an organism.
True.
Enzymes determine structures and functions from cellular to whole-organism level.
Describe the lock and key model of enzyme action.
The lock and key model uses the idea that only a substrate that exactly fits an enzyme's active site can bind to the enzyme.
The enzyme catalyses the reaction and releases the products.
Describe the induced fit model of enzyme action.
The induced fit model involves the enzyme changing shape slightly after the substrate binds to the active site, which bends and distorts bonds in the substrate.
The active site is now complementary to the substrate.
The enzyme catalyses the reaction and releases the product.
How is the shape of an enzyme's active site determined?
The shape of an enzyme's active site is determined by the protein's tertiary structure.
Hence, each different enzyme has a different tertiary structure as they have a different active site.
What will happen to an enzyme if the tertiary structure is altered?
If the tertiary structure is altered, the shape of the active site will change.
The substrate won't fit into the active site, so the reaction would not be catalysed.
Describe how the enzyme concentration affects the rate of reaction.
The higher the enzyme concentration, the more likely a substrate molecule is to collide with an enzyme.
Hence, enzyme-substrate complexes are more likely to be formed, increasing the rate of reaction.
Describe how the substrate concentration affects the rate of reaction.
The higher the substrate concentration, the more likely a substrate molecule is to collide with an enzyme.
This is only true up to the saturation point, where all the active sites are full.
As substrate concentration decreases with time, the rate of reaction will decrease over time.
What is a competitive inhibitor?
A competitive inhibitor molecule has a similar shape to substrate molecules.
They bind to the active site, but no reaction takes place.
Describe how the concentration of competitive inhibitors affects the rate of reaction.
The higher the concentration of the competitive inhibitor is, the more likely it is to block the active site.
Hence, barely any of the substrate will reach the enzyme, so little enzyme-substrate complexes are formed.
What do non-competitive inhibitors do to enzymes?
A non-competitive inhibitor molecule binds to the enzyme away from its active site.
The active site changes shape as a result.
Describe how the concentration of non-competitive inhibitors affects the rate of reaction.
The higher the concentration of the non-competitive inhibitor, the more enzymes with an altered active site.
Hence, the substrates cannot bind to the active site.
Describe how the pH affects the rate of reaction.
All enzymes have an optimum pH value.
Above and below the optimum pH, H+ and OH- ions (from the acids and alkalis) can destroy the ionic bonds and hydrogen bonds in the enzyme's tertiary structure.
This changes the shape of the active site, so the enzyme is denatured.
Describe how the temperature affects the rate of reaction.
As the temperature increases, the kinetic energy of the enzymes increases.
This increases the frequency of collisions between the enzymes and the substrates.
The collisions are more energetic, so a reaction is more likely.
What happens to an enzyme if the temperature is too high?
If the temperature is too high, the bonds in the tertiary structure break.
This changes the shape of the active site, so the enzyme is denatured.
What are the two important information-carrying molecule?
Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are the two important information-carrying molecules.
What does DNA do in all living cells?
In all living cells, DNA holds genetic information.
What does RNA do in all living cells?
In all living cells, RNA transfers genetic information from DNA to the ribosomes.
What are ribosomes formed from?
Ribosomes are formed from RNA and proteins.
What are both DNA and RNA polymers of?
Both DNA and RNA are polymers of nucleotides.
What is each nucleotide formed from?
Each nucleotide is formed from a pentose, a nitrogen-containing organic base and a phosphate group.
What are the components of a DNA nucleotide?
The components of a DNA nucleotide are deoxyribose, a phosphate group and one of the organic bases adenine, cytosine, guanine or thymine.
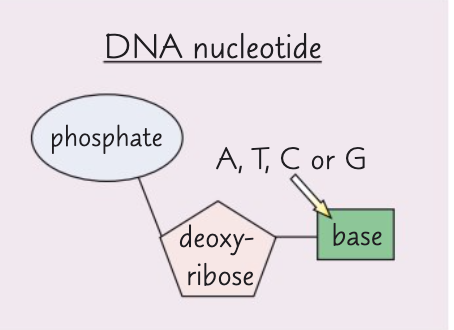
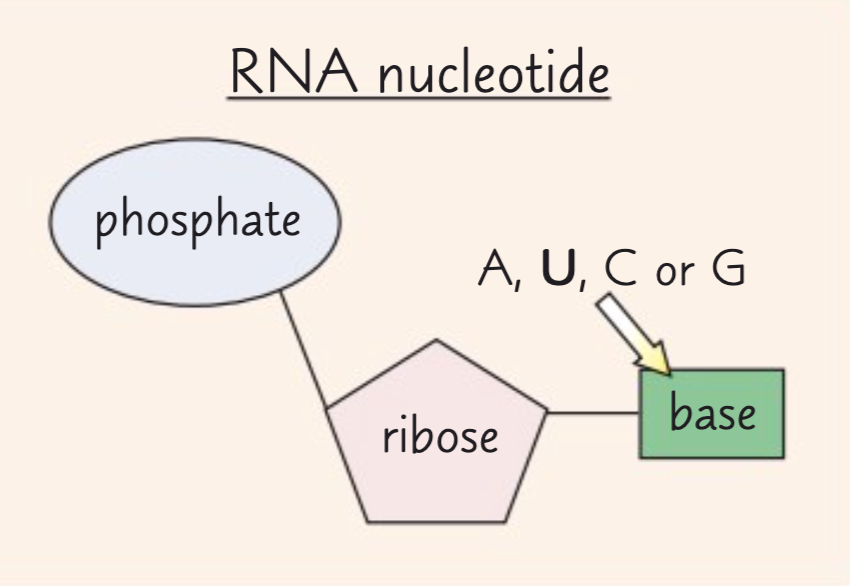
What are the components of an RNA nucleotide?
The components of an RNA nucleotide are ribose, a phosphate group and one of the organic bases adenine, cytosine, guanine or uracil.
How is a phosphodiester bond formed?
A phosphodiester bond is formed by a condensation reaction between two nucleotides.
The phosphate group of one nucleotide bonds with the sugar of the other.
Describe the structure of a DNA molecule.
A DNA molecule is a double helix with two antiparallel polynucleotide chains held together by hydrogen bonds between specific complementary base pairs.
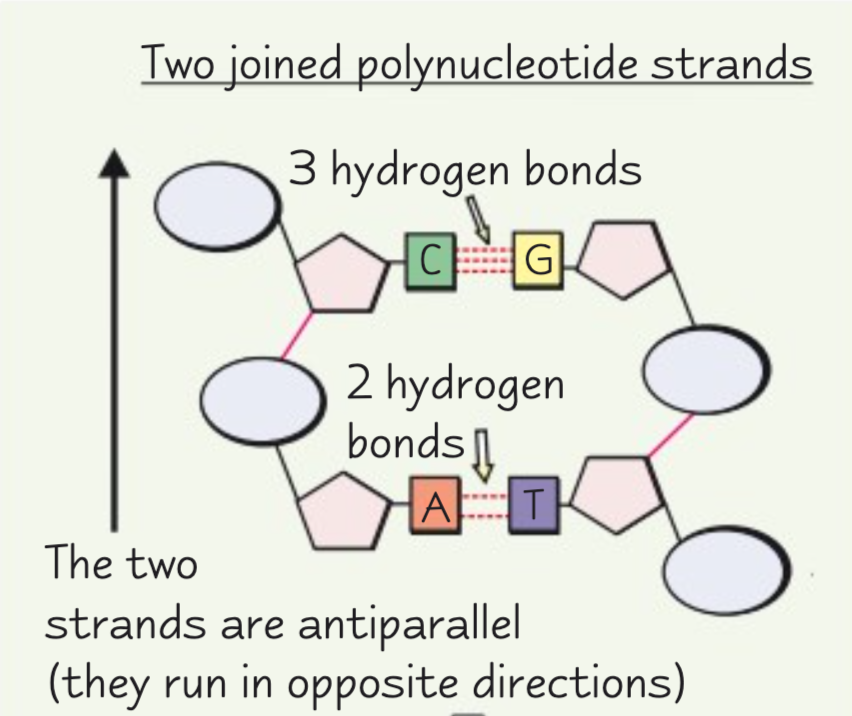
How many hydrogen bonds are between each complementary base pair?
There are two hydrogen bonds between adenine and thymine.
There are three hydrogen bonds between guanine and cytosine.
Give six ways the structure of DNA is related to its function.
DNA has a sugar-phosphate backbone which provides strength to protect bases and hydrogen bonds.
DNA is a large molecule so it can store lots of information
DNA has a helical shape, which coils so it is compact.
The base sequence in DNA codes for amino acids.
DNA is double-stranded so replication can occur semi-conservatively.
There are weak hydrogen bonds for strand separation.
Describe the structure of an RNA molecule.
An RNA molecule is a relatively short polynucleotide chain.
Why did many scientists doubt that DNA carried the genetic code?
Many scientists doubted that DNA carried the genetic code due to its relative simplicity.
How is genetic continuity ensured between generations of cells?
Genetic continuity is ensured between generations of cells through the semi-conservative replication of DNA.
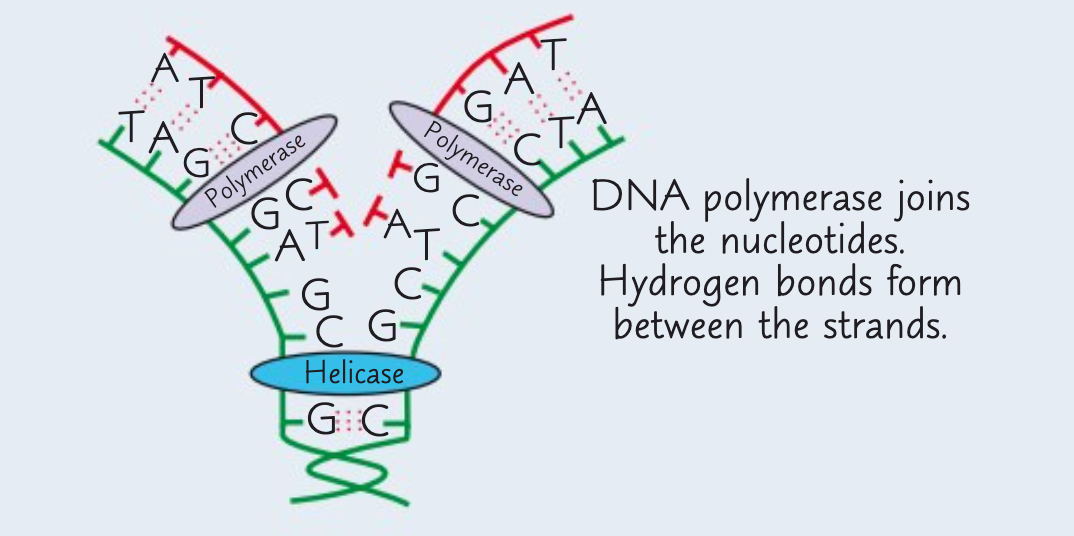
What is the first stage of semi-conservative replication of DNA?
The first stage is the unwinding of the double helix.
The enzyme DNA helicase breaks the hydrogen bonds between complementary bases on the two polynucleotide strands. This gives two single strands.
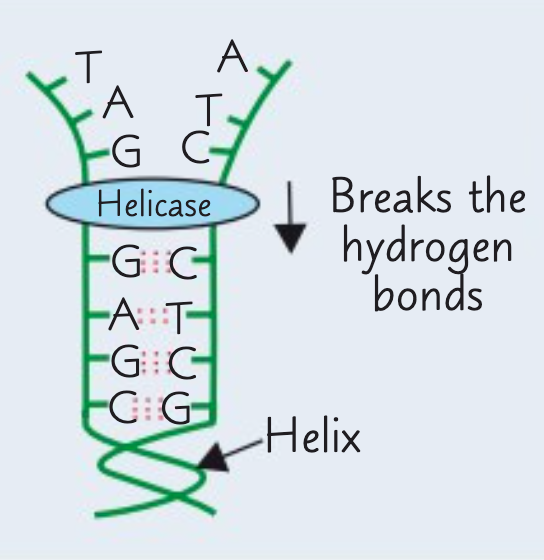
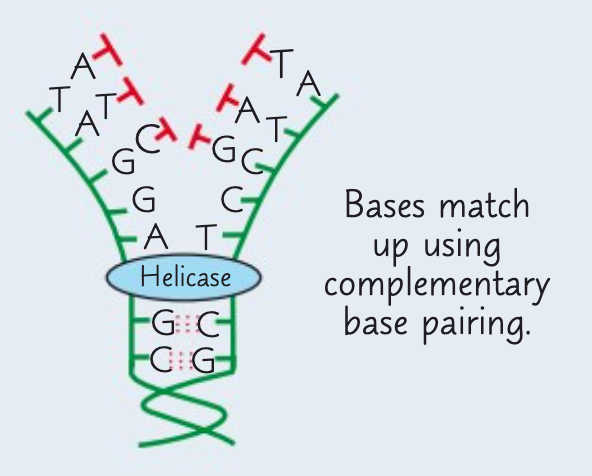
What is the second stage of semi-conservative replication of DNA?
The second stage is the attraction of new free floating DNA nucelotides to complementary exposed bases on template strands.
What is the third stage of semi-conservative replication of DNA?
The third stage is the joining of adjacent nucleotides.
This is done by the enzyme DNA polymerase which catalyses condensation reactions between the nucleotides.
Hydrogen bonds form between the bases.
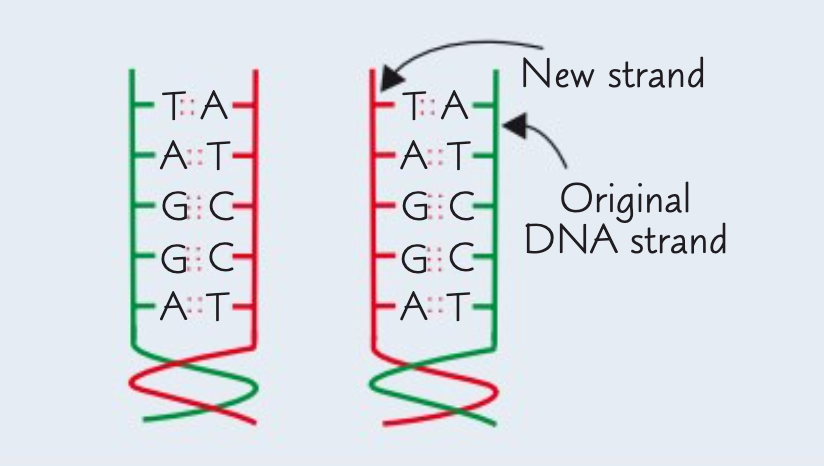
What is the result of semi-conservative replication of DNA?
The result is two identical copies of DNA molecules.
Each new DNA molecule contains one strand from the original DNA molecule and one new strand.
What are the names of each end of a DNA strand? How are these different?
One end of a DNA strand is called the 3' end and the other is called the 5' end.
They have different structures.
What is the active site of DNA polymerase complementary to?
The active site of DNA polymerase complementary to the 3' end of the newly forming DNA strand.
This means the enzyme can only add nucleotides at the 3' end.
How is the movement of DNA polymerase determined by its active site?
DNA polymerase moves down the template strand in a 3' to 5' direction. Hence, the new strand is made in a 5' to 3' direction.
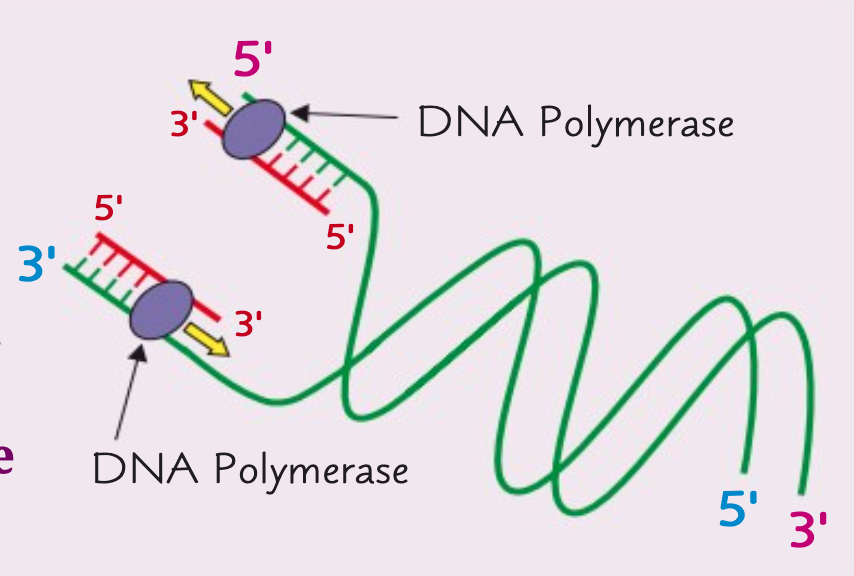
Why do the DNA polymerase enzymes move in opposite directions?
The DNA polymerase enzymes move in opposite directions because the polynucleotide strands are antiparallel.
This means the 3' ends are on opposite ends.
DNA polymerase can only move in a 3' to 5' direction.
What did Meselson's and Stahl's experiment use?
Meselson's and Stahl's experiment use two isotopes of nitrogen, heavy nitrogen and light nitrogen.
This was to validate the Watson-Crick model.
Describe the first step of Meselson's and Stahl's experiment.
The first step involved two samples of bacteria being grown.
One in a nutrient broth with light nitrogen, and one in a broth with heavy nitrogen.
Each isotope became part of each bacteria sample's DNA.
Describe the second step of Meselson's and Stahl's experiment.
The second step involved taking a sample of DNA from each batch of bacteria.
This was spun in a centrifuge - the DNA from the heavy nitrogen settled lower than the DNA from the light nitrogen.
Describe the third step of Meselson's and Stahl's experiment.
The third step involved placing the bacteria grown in the heavy nitrogen in a broth with only light nitrogen.
They were left for one round of DNA replication, then another DNA sample was taken and spun in the centrifuge.
If DNA replication was conservative, what would show when the new DNA sample was spun in the centrifuge?
If DNA replication was conservative, the original heavy DNA, still together, would settle at the bottom.
The new light DNA would settle at the top.
If DNA replication was semi-conservative, what would show when the new DNA sample was spun in the centrifuge?
If DNA replication was semi-conservative, the new DNA molecules would contain one strand of the old DNA with heavy nitrogen, and one strand of the new DNA with light nitrogen.
So the DNA would settle in the middle.
What were the results of Meselson's and Stahl's experiment?
Meselson's and Stahl's experiment resulted in the DNA settling in the middle of the tube.
This showed the DNA molecules had a mixture of heavy and light nitrogen.
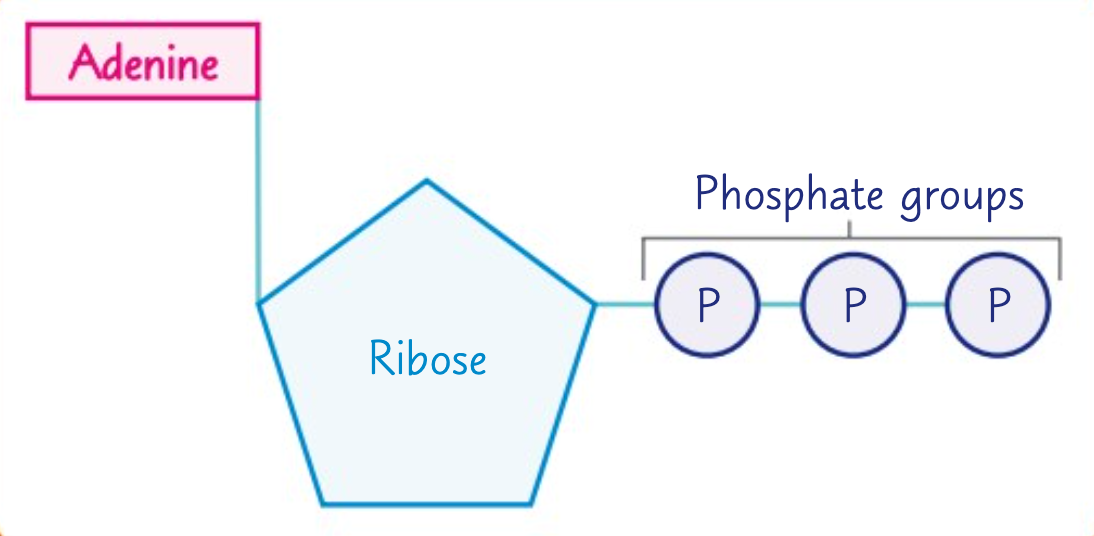
Which biological molecule is a nucleotide derivative?
A single molecule of adenosine triphosphate (ATP) is a nucleotide derivative.
What is ATP formed from?
ATP is formed from a molecule of ribose, a molecule of adenine, and three phosphate groups.