Lecture 9 - enzymes (mainly chymotrypsin mechanism)
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
Mechanisms of Enzyme Catalysis
Enzymes use their AA side-chains to participate in
the chemical transformation. We will use the classic
example of chymotrypsin as an illustrative example
Two main catalytic players:
Acidic residues:
These donate H⁺ and accept electrons (like carboxylic acids - e.g., Asp, Glu)Basic residues:
These accept H⁺ and donate electrons (like amines - e.g., His, Lys)
what is Nucleophiles ("nucleus-loving"):?
Electron-rich species that donate electrons. and is attracted to electron-poor regions — typically areas with a partial positive charge, like the carbonyl carbon in a peptide bond
Examples:
Negatively charged oxygen (like deprotonated OH or COO⁻)
Carbanions, amines, imidazole (His side chain), hydroxide ion
electron donor (attacker)
what are Electrophiles ("electron-loving"):?
Electron-poor species that accept electrons.
Examples:
Carbon of a carbonyl group (C=O)
Protonated imine, phosphorus atoms in phosphate groups, protons (H⁺)
electron acceptor (target)
what is a key structural component of chymotrypsin mechanism? (2)
Catalytic triad:
Ser195
His57
Asp102
These three amino acids work together to catalyze the cleavage of peptide bonds.
Aromatic side chain pocket:
This is a hydrophobic pocket that binds the aromatic side chains (like Phe, Trp, Tyr) of the substrate. This specificity explains chymotrypsin’s selectivity.
what is chymotrypsin?
Chymotrypsin is a serine protease that cleaves peptide bonds after aromatic residues (Trp, Tyr, Phe). It uses a catalytic triad of amino acids: Ser195, His57, and Asp102.
Role of ASP102?
Aspartate 102 (Asp102) — The stabilizer
Function: Helps stabilize and orient His57.
It forms a hydrogen bond with His57, holding it in position and helping it accept protons more easily.
Without Asp102, His57 wouldn’t be nearly as effective.
Role of His57
Histidine 57 (His57) — The proton shuttle
Function: Acts as a general base and acid.
First, it removes a proton from Ser195, helping activate it.
Later, it gives that proton to the leaving group (and later to water), shuttling protons as needed throughout the reaction.
Role of Ser195?
Serine 195 (Ser195) — The attacker
Function: Acts as a nucleophile — meaning it attacks the carbonyl carbon of the peptide bond.
How: Its –OH group normally isn’t very reactive, but His57 pulls away its proton, converting it into a strong O⁻ nucleophile.
what are the 7 steps for the chymotrypsin mechanism?
Substrate Binding
Formation of the First Tetrahedral Intermediate
Cleavage of the Peptide Bond (Acylation)
Water Molecule Enters
Formation of the Second Tetrahedral Intermediate
Collapse of the Intermediate and Product Release (Deacylation)
Enzyme Returns to Original State
explain step 1
Substrate Binding
The substrate (a polypeptide) enters the active site.
The aromatic side chain of the target residue fits into a hydrophobic pocket, positioning the peptide bond for cleavage.
The side chains of His57, Asp102, and Ser195 form the catalytic triad.
What causes the pKa of His57 to change upon substrate binding?
Compression of the bond between Asp102 and His57
Explanation: This physical compression alters the electrostatic environment, significantly raising the pKa of His57 above 12
explain step 2
Formation of the First Tetrahedral Intermediate
His57 acts as a base, pulling a proton off Ser195.
Ser195 becomes a strong nucleophile (O⁻) and attacks the carbonyl carbon of the peptide bond.
This forms a high-energy tetrahedral intermediate.
The oxyanion (O⁻ on the carbonyl oxygen) is stabilized in the oxyanion hole.
what is a tetrahedral intermediate
A tetrahedral intermediate is a short-lived, high-energy molecule that forms when a nucleophile attacks a carbonyl carbon, temporarily breaking the carbon's double bond to oxygen and turning the carbon into a tetrahedral geometry (bonded to four different atoms/groups).
explain step 3
Cleavage of the Peptide Bond (Acylation)
His57 now donates the proton it grabbed earlier (acts as an acid). to the nitrogen atom of the scissle bond
The peptide bond breaks — the C-terminal fragment leaves.
The N-terminal fragment stays covalently attached to Ser195 → called the acyl-enzyme intermediate.
explain step 4
Water Molecule Enters
A water molecule now enters the active site.
His57 acts as a base again, abstracting a proton from water.
This activates water to become a nucleophile (OH⁻).
explain step 5
Formation of the Second Tetrahedral Intermediate
The OH⁻ from water attacks the carbonyl carbon of the acyl-enzyme intermediate.
This creates a second tetrahedral intermediate, again stabilized by the oxyanion hole.
explain step 6
Collapse of the Intermediate and Product Release (Deacylation)
The intermediate collapses.
His57 donates a proton to regenerate Ser195's OH, breaking the bond between Ser195 and the substrate.
The N-terminal peptide is released.
The N-terminal product is now free to depart from the active site and the catalytic triad is ready to receive another substrate molecule.
explain step 7
Enzyme Returns to Original State
All active site residues are restored to their original state.
Chymotrypsin is ready to catalyze another reaction.
Why on earth study protease mechanism (and many
other enzymes) to such a degree?
These mechanistic studies provide fundamental
knowledge for understanding the molecular basis of life,
modern drug development, medicine and agriculture
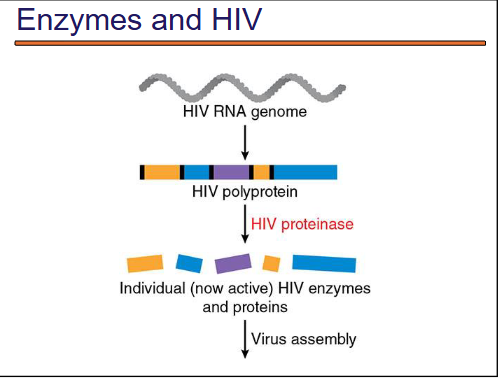
how does HIV carry its gene info?
The HIV virus carries its genetic information in the form of RNA.
what is a HIv polyprotein?
An HIV polyprotein is a large protein that is initially synthesized as a single, long chain from the HIV RNA genome. It contains the sequences for multiple viral proteins all joined together, rather than being made as separate individual proteins.
what happens when HIV infects a host?
When HIV infects a host cell, its RNA genome is reverse-transcribed into DNA and integrated into the host genome.
The host cell’s machinery transcribes and translates this viral DNA into one long polypeptide chain — the polyprotein.
what happens to the polyprotein?
The polyprotein cannot function as-is. It must be cleaved (cut) into individual proteins.
This is done by HIV protease, an enzyme that cuts at specific sites in the polyprotein.
what happens after cleavage of the polyprotein?
Once cleaved, they become into individual functional proteins. the individual proteins become active enzymes and structural components.
With the now-functional proteins, the virus assembles into infectious particles.
Now can perform their roles in:
viral replication
genome integration
viral assembly
HIV infection summarized photo
what is Viracept?
A inhibitor that binds to HIV proteases which is used for HIV treatment
Why is viracept for important?
HIV protease is essential for viral replication—it cleaves the HIV polyprotein into functional units.
Inhibitors like Viracept block the active site of the protease so it can’t cleave the polyprotein.
This prevents the formation of mature, infectious virus particles.
how many enzymes use metal cofactors as enzymes?
About 1/3 of all enzymes require metal ions (like Zn²⁺, Fe²⁺, Mg²⁺) to function.
role of metal in catlysis?
Stabilize negative charges in the transition state (which is usually high energy and unstable).
Help orient the substrate properly in the active site.
Participate in redox reactions, accepting or donating electrons.
what does Carboxypeptidase do?
Carboxypeptidase catalyzes the hydrolysis of peptide bonds at the C-terminal end of proteins, releasing one amino acid at a time.
what does the enzyme Carboxypeptidase use and why? (3)
This enzyme uses a metal ion (usually Zn²⁺) to catalyze the hydrolysis of peptide bonds.
It helps cleave amino acids from the C-terminus of peptides.
The metal ion polarizes the carbonyl group (C=O) and activates a water molecule to act as a nucleophile for bond cleavage.
what does zinc do in active sites?
Zinc is a metal cofactor.
It stabilizes negative charges on oxygen atoms (like in carbonyl groups).
Helps form an oxyanion-like transition state, similar to the role of the oxyanion hole in chymotrypsin.
full mechanism of carboxypeptidase?
