2.1 Thermochemistry
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Enthalpy change of reaction
is the enthalpy change when the molar quantities of reactants, as stated in the balanced equation, react under standard conditions
Enthalpy change of combustion
is the energy released when one mole of a substance burns completely in oxygen under standard conditions.
Enthalpy change of formation
is the enthalpy change when one mole of a compound is formed from its elements, in their standard states, under standard conditions
Bond enthalpy
is the energy required to break one mole of that particular bond in the gaseous state.
Hess’ Law
that the enthalpy change converting reactants to products is the same regardless of the route taken provided the initial and final conditions are the same.
Q=
mcΔT
ΔH =
-Q/n
Exothermic
there is a net transfer of energy from the system to the surroundings. |
Endothermic
there is a net transfer of energy from the surroundings to the system. |
Sign for exothermic
negative
Sign for endothermic
positive
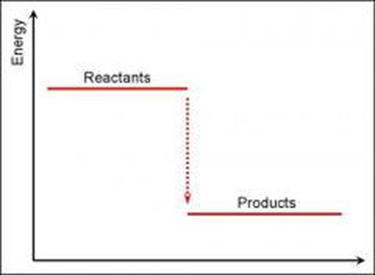
Exothermic energy level diagram

Endothermic energy level diagram
Specific heat capacity
the energy needed to raise the temperature of 1g of the material by 1K |
Standard conditions are
1 mol dm-3 100kPa 298K |
Calorimetry
the measurement of energy changes of a system by measuring their effect on surroundings. |